The Potential of Polyhydroxyalkanoate Production from Food Wastes
Applied Food Biotechnology,
Vol. 6 No. 1 (2019),
2 January 2019
,
Page 7-18
https://doi.org/10.22037/afb.v6i1.22542
Abstract
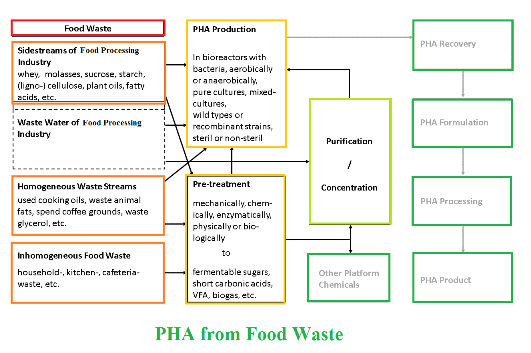
Background and objective: Over 1 billion tons of foods are wasted every year (not consumed by humans or animals). Most of this waste ends up in landfills. As the global population increases, mankind must look for more sustainable means of living. A recently popular idea is the use of organic wastes as carbon feedstocks for fermentation that produces value added products. Polyhydroxyalkanoates are a family of bio-based, biodegradable polymers that can be produced in large quantities using food and food processing wastes as the main feedstocks. In many cases, biocatalysts have been engineered to efficiently use these waste compounds to produce large quantities of useful intracellular polyhydroxyalkanoates.
Results and conclusion: In the current study, various polyhydroxyalkanoates were produced; each with different thermal and mechanical characteristics useful for different applications. If polyhydroxyalkanoate production facilities are established next to food waste accumulation sites (e.g., large landfills), potentials for the economical and sustainable polyhydroxyalkanoate production sound promising.
Conflict of interest: The authors declare no conflict of interest.
- ▪ Biopolymers ▪ Carbon feedstock ▪ Fermentation ▪ Food waste ▪ Polyhydroxyalkanoate
How to Cite
References
Steinbüchel A. Polyhydroxyalkanoic acids. In: Biomaterials, Novel Materials. Springer; 1991. p. 123–213. doi:10.1007/978-1-349-11167-1_3.
Brigham CJ, Budde CF, Holder JW, Zeng Q, Mahan AE, Rha CK, et al. Elucidation of β-oxidation pathways in Ralstonia eutropha H16 by examination of global gene expression. J Bacteriol. 2010;192(20):5454–64. doi:10.1128/JB.00493-10.
Riedel SL, Bader J, Brigham CJ, Budde CF, Yusof ZAM, Rha C, et al. Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol Bioeng. 2012;109(1):74–83. doi:10.1007/s00253-013-5430-8.
Riedel SL, Lu J, Stahl U, Brigham CJ. Lipid and fatty acid metabolism in Ralstonia eutropha: Relevance for the biotechnological production of value-added products. Appl Microbiol Biotechnol. 2014;98(4):1469–83. doi:10.1007/s00253-013-5430-8.
Sudesh K, Abe H, Doi Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog Polym Sci. 2000; doi:10.1016/S0079-6700(00)00035-6.
Reinecke F, Steinbüchel A. Ralstonia eutropha strain H16 as model organism for PHA metabolism and for biotechnological production of technically interesting biopolymers. J Mol Microbiol Biotechnol. 2008;16(1–2):91–108. doi:10.1159/000142897.
Wilde E. Untersuchungen über Wachstum und Speicherstoffsynthese von Hydrogenomonas. Arch für Mikrobiol. 1962;43(2):109–37. doi:10.1007/BF00406429.
James BW, Mauchline WS, Dennis PJ, Keevil W, Wait R, Keevil CW. Poly-3-Hydroxybutyrate in Legionella pneumophila , an Energy Source for Survival in Low-Nutrient Environments Poly-3-Hydroxybutyrate in Legionella pneumophila , an Energy Source for Survival in Low-Nutrient Environments. Appl Environ Microbiol. 1999;65(2):822–7.
Qi Q, Rehm BHA. Polyhydroxybutyrate biosynthesis in Caulobacter crescentus: Molecular characterization of the polyhydroxybutyrate synthase. Microbiology. 2001;147(12):3353–8. doi:10.1099/00221287-147-12-3353.
Rehm BHA, Krüger N, Steinbüchel A. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. J Biol Chem. 1998;273(37):24044–24051. doi: 10.1074/jbc.273.37.24044.
Hoffmann N, Steinbüchel A, Rehm BHA. The Pseudomonas aeruginosa phaG gene product is involved in the synthesis of polyhydroxyalkanoic acid consisting of medium-chain-length constituents from non-related carbon sources. FEMS Microbiol Lett. 2000;184(2):253–9. doi:10.1016/S0378-1097(00)00047-1.
Hoffmann N, Steinbuchel A, Rehm BHA. Homologous functional expression of cryptic phaG from Pseudomonas oleovorans establishes the transacylase-mediated polyhydroxyalkanoate biosynthetic pathway. Appl Microbiol Biotechnol. 2000;54(5):665–70. doi:10.1007/s002530000441.
Rehm BHA. Polyester synthases: natural catalysts for plastics. Biochem J [Internet]. 2003;376(1):15–33. doi:10.1042/bj20031254.
Wong YM, Brigham CJ, Rha CK, Sinskey AJ, Sudesh K. Biosynthesis and characterization of polyhydroxyalkanoate containing high 3-hydroxyhexanoate monomer fraction from crude palm kernel oil by recombinant Cupriavidus necator. Bioresour Technol. 2012;121:320–7. doi:10.1042/bj20031254.
Fukui T, Abe H, Doi Y. Engineering of Ralstonia eutropha for production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from fructose and solid-state properties of the copolymer. Biomacromolecules. 2002;3(3):618–24. doi:10.1021/bm0255084.
Emadian SM, Onay TT, Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017;59:526–36. doi:10.1016/j.wasman.2016.10.006.
Keshavarz T, Roy I. Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol. 2010;13(3):321–6. doi:10.1016/j.mib.2010.02.006.
Sudesh K, Bhubalan K, Chuah JA, Kek YK, Kamilah H, Sridewi N, et al. Synthesis of polyhydroxyalkanoate from palm oil and some new applications. Appl Microbiol Biotechnol. 2011;89(5):1373–86. doi:10.1007/s00253-011-3098-5.
Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38(8):2434–46. doi:10.1039/b812677c.
Brigham, Sinskey. Applications of Polyhydroxyalkanoates in the Medical Industry. Int J Biotechnol Wellness Ind. 2012;1(1):53–60. doi:10.6000/1927-3037.2012.01.01.03.
Boyandin AN, Kazantseva EA, Varygina DE, Volova TG. Constructing Slow-Release Formulations of Ammonium Nitrate Fertilizer Based on Degradable Poly(3-hydroxybutyrate). J Agric Food Chem. 2017;65(32):6745–52. doi:10.1021/acs.jafc.7b01217.
Madkour MH, Heinrich D, Alghamdi MA, Shabbaj II, Steinbüchel A. PHA recovery from biomass. Vol. 14, Biomacromolecules. 2013. p. 2963–72. doi:10.1021/bm4010244.
Koller M, Niebelschütz H, Braunegg G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) biopolyesters from surrounding biomass. Eng Life Sci. 2013;13(6):549–62. doi:10.1002/elsc.201300021.
Ong SY, Kho H-P, Riedel SL, Kim S-W, Gan C-Y, Taylor TD, et al. An integrative study on biologically recovered polyhydroxyalkanoates (PHAs) and simultaneous assessment of gut microbiome in yellow mealworm. J Biotechnol. 2017;265:31–9. doi:10.1016/j.jbiotec.2017.10.017.
Ong SY, Zainab-L I, Pyary S, Sudesh K. A novel biological recovery approach for PHA employing selective digestion of bacterial biomass in animals. Appl Microbiol Biotechnol. 2018;102(5):2117–27. doi:10.1007/s00253-018-8788-9.
European Bioplastics. Bioplastics market data 2017 - Global production capacities of bioplastics 2017-2022. 2017;4. Available from: http://docs.european-bioplastics.org/publications/market_data/2017/Report_Bioplastics_Market_Data_2017.pdf
Możejko-Ciesielska J, Kiewisz R. Bacterial polyhydroxyalkanoates: Still fabulous? Vol. 192, Microbiological Research. 2016. p. 271–82. doi:10.1016/j.micres.2016.07.010.
Ravindran R, Jaiswal AK. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016;34(1):58–69. doi:10.1016/j.tibtech.2015.10.008.
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN. Conversion of agricultural feedstock and coproducts into poly(hydroxyalkanoates). Appl Microbiol Biotechnol. 2006;71(6):783–9. doi:10.1007/s00253-006-0451-1.
FitzPatrick M, Champagne P, Cunningham MF, Whitney RA. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol. 2010;101(23):8915–22. doi:10.1016/j.biortech.2010.06.125.
Fava F, Totaro G, Diels L, Reis M, Duarte J, Carioca OB, et al. Biowaste biorefinery in Europe: Opportunities and research & development needs. N Biotechnol. 2015;32(1):100–8. doi:10.1016/j.nbt.2013.11.003.
Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, Hamilton R, et al. How biotech can Transformation biofuels. 2008;26(2):169–72. doi: 10.1038/nbt0208-169
Salgaonkar BB, Bragança JM. Utilization of Sugarcane Bagasse by Halogeometricum borinquense Strain E3 for Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Bioengineering. 2017;4(2):50. doi:10.3390/bioengineering4020050.
Kourmentza C, Costa J, Azevedo Z, Servin C, Grandfils C, De Freitas V, et al. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour Technol. 2018;247:829–37. doi:10.1016/j.biortech.2017.09.138.
Obruca S, Marova I, Snajdar O, Mravcova L, Svoboda Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol Lett. 2010;32(12):1925–32. doi:10.1007/s10529-010-0376-8.
Benesova P, Kucera D, Marova I, Obruca S. Chicken feather hydrolysate as an inexpensive complex nitrogen source for PHA production by Cupriavidus necator on waste frying oils. Lett Appl Microbiol. 2017;65(2):182–8. doi:10.1111/lam.12762.
Kamilah H, Al-Gheethi A, Yang TA, Sudesh K. The Use of Palm Oil-Based Waste Cooking Oil to Enhance the Production of Polyhydroxybutyrate [P(3HB)] by Cupriavidus necator H16 Strain. Arab J Sci Eng. 2018;43(7):3453–63. doi:10.1007/s13369-018-3118-1.
Obruca S, Benesova P, Kucera D, Petrik S, Marova I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. N Biotechnol. 2015;32(6):569–74. doi:10.1016/j.nbt.2015.02.008.
Kovalcik A, Obruca S, Marova I. Valorization of spent coffee grounds: A review. Food Bioprod Process. 2018;110:104–19. doi:10.1016/j.fbp.2018.05.002.
Obruca S, Benesova P, Petrik S, Oborna J, Prikryl R, Marova I. Production of polyhydroxyalkanoates using hydrolysate of spent coffee grounds. Process Biochem. 2014;49(9):1409–14. doi:10.1016/j.procbio.2014.05.013.
Bhatia SK, Kim JH, Kim MS, Kim J, Hong JW, Hong YG, et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst Eng. 2018;41(2):229–35. doi:10.1007/s00449-017-1861-4.
Riedel SL, Jahns S, Koenig S, Bock MC, Brigham CJ, Bader J, et al. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J Biotechnol. 2015;214:119–27. doi:10.1016/j.jbiotec.2015.09.002.
Koller M, Braunegg G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and economic fashion. EuroBiotech J. 2018;2(2):89–103. doi:10.2478/ebtj-2018-0013.
Atasoy M, Owusu-Agyeman I, Plaza E, Cetecioglu Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour Technol. 2018;0–1. doi:10.1016/j.biortech.2018.07.042.
Liu H, Han P, Liu H, Zhou G, Fu B, Zheng Z. Full-scale production of VFAs from sewage sludge by anaerobic alkaline fermentation to improve biological nutrients removal in domestic wastewater. Bioresour Technol. 2018;260:105–14. doi:10.1016/J.BIORTECH.2018.03.105.
Yuan H, Chen Y, Zhang H, Jiang S, Zhou Q, Gu G. Improved Bioproduction of Short-Chain Fatty Acids (SCFAs) from Excess Sludge under Alkaline Conditions. Environ Sci Technol. 2006 Mar;40(6):2025–9. doi:10.1021/es052252b.
Wang K, Yin J, Shen D, Li N. Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: Effect of pH. Bioresour Technol. 2014;161:395–401. doi:10.1016/j.biortech.2014.03.088.
Domingos JMB, Martinez GA, Scoma A, Fraraccio S, Kerckhof FM, Boon N, et al. Effect of operational parameters in the continuous anaerobic fermentation of cheese whey on titers, yields, productivities, and microbial community structures. ACS Sustain Chem Eng. 2017;5(2):1400–7. doi:10.1021/acssuschemeng.6b01901.
Domingos JMB, Puccio S, Martinez GA, Amaral N, Reis MAM, Bandini S, et al. Cheese whey integrated valorisation: Production, concentration and exploitation of carboxylic acids for the production of polyhydroxyalkanoates by a fed-batch culture. Chem Eng J. 2018;336:47–53. doi:10.1016/j.cej.2017.11.024.
Arslan D, Zhang Y, Steinbusch KJJ, Diels L, Hamelers HVM, Buisman CJN, et al. In-situ carboxylate recovery and simultaneous pH control with tailor-configured bipolar membrane electrodialysis during continuous mixed culture fermentation. Sep Purif Technol. 2017;175:27–35. doi:10.1016/j.seppur.2016.11.032.
Valentino F, Morgan-Sagastume F, Campanari S, Villano M, Werker A, Majone M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. N Biotechnol. 2017;37:9–23. doi:10.1016/j.nbt.2016.05.007.
Amulya K, Jukuri S, Venkata Mohan S. Sustainable multistage process for enhanced productivity of bioplastics from waste remediation through aerobic dynamic feeding strategy: Process integration for up-scaling. Bioresour Technol. 2015;188:231–9. doi:10.1016/j.biortech.2015.01.070.
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N Biotechnol. 2017;37:24–38. doi:10.1016/j.nbt.2016.05.001.
Anderson JH, Anderson DH. Producing resins from organic waste products. Google Patents; 2016. U.S. Patent Application No. 14/947,873.
Venus J, Fiore S, Demichelis F, Pleissner D. Centralized and decentralized utilization of organic residues for lactic acid production. J Clean Prod. 2018;172:778–85. doi:10.1016/j.jclepro.2017.10.259.
Kwan TH, Hu Y, Lin CSK. Techno-economic analysis of a food waste valorisation process for lactic acid, lactide and poly(lactic acid) production. J Clean Prod. 2018;181:72–87. doi:10.1016/j.jclepro.2018.01.179.
Lin CSK, Pfaltzgraff LA, Herrero-Davila L, Mubofu EB, Abderrahim S, Clark JH, et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ Sci. 2013;6(2):426–64. doi:10.1039/c2ee23440h.
Nielsen C, Rahman A, Rehman AU, Walsh MK, Miller CD. Food waste conversion to microbial polyhydroxyalkanoates. Microb Biotechnol. 2017;10(6):1338–52. doi:10.1111/1751-7915.12776.
Colombo B, Favini F, Scaglia B, Sciarria TP, D’Imporzano G, Pognani M, et al. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol Biofuels. 2017;10(1):201. doi:10.1186/s13068-017-0888-8.
Uçkun Kiran E, Liu Y. Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel. 2015;159:463–9. doi:10.1016/j.fuel.2015.06.101.
Huang H, Singh V, Qureshi N. Butanol production from food waste: a novel process for producing sustainable energy and reducing environmental pollution. Biotechnol Biofuels. 2015;8(1):1–12. doi:10.1186/s13068-015-0332-x.
Haque MA, Kachrimanidou V, Koutinas A, Lin CSK. Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J Biotechnol. 2016;231:55–64. doi:10.1016/j.jbiotec.2016.05.003.
Izumiya M, Tamaru M, Nonaka A. Status and Issues of Exchange Systems for Dried Food Waste and Vegetables in the Recycling of Household Food Waste. Bull Fac Agric Life Sci Hirosaki Univ No. 2018;20:1–5.
Hassan MA, Nawata O, Shirai Y, Rahman NAA, Yee PL, Ariff A Bin, et al. A Proposal for Zero Emission from Palm Oil Industry Incorporating the Production of Polyhydroxyalkanoates from Palm Oil Mill Effluent. J Chem Eng JAPAN. 2002; doi:10.1252/jcej.35.9.
Huschner F, Grousseau E, Brigham CJ, Plassmeier J, Popovic M, Rha C, et al. Development of a feeding strategy for high cell and PHA density fed-batch fermentation of Ralstonia eutropha H16 from organic acids and their salts. Process Biochem. 2015;50(2):165–72. doi:10.1016/j.procbio.2014.12.004.
Rodriguez-Perez S, Serrano A, Pantión AA, Alonso-Fariñas B. Challenges of scaling-up PHA production from waste streams. A review. J Environ Manage. 2018;205:215–30. doi:10.1016/j.jenvman.2017.09.083.
- Abstract Viewed: 1863 times
- PDF Downloaded: 2021 times