Interconnection of Waste Chicken Feather Biodegradation and Keratinase and mcl-PHA Production Employing Pseudomonas putida KT2440
Applied Food Biotechnology,
Vol. 6 No. 1 (2019),
2 January 2019
,
Page 83-90
https://doi.org/10.22037/afb.v6i1.21429
Abstract
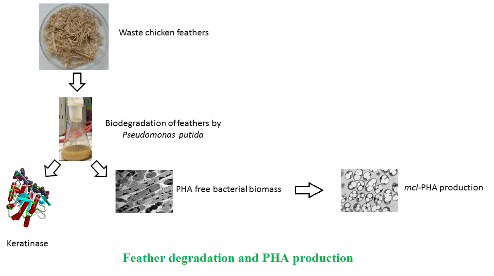
Background and objective: Waste chicken feather is an important waste product of the poultry processing industry and is annually produced in substantial amounts. Hence, wise management of this waste is desirable. In this work we aimed at feathers biodegradation by a selected bacterial strain capable of utilizing chicken feathers as sole carbon source Pseudomonas putida KT2440. To utilize feather, the bacterial culture excrete keratinase, which can be easily isolated after biodegradation process and which, therefore, represents an interesting side product of the intended technology. Moreover, bacterial culture of employed for feather degradation is also capable of mcl-PHA accumulation.
Materials and methods: Bacterial culture of Pseudomonas putida KT2440 was cultivated in presence of waste chicken feathers as the only carbon source; during the cultivation keratinase activity and biomass growth were monitored. Metabolically active biomass after feather degradation was used for mcl-PHA production.
Results and conclusion: During cultivation on waste feathers, bacteria did not accumulate detectable amounts of medium-chain length polyhydroxyalkanoate (mcl-PHA); nevertheless, when metabolically active bacterial cells after feather biodegradation were transferred into nitrogen limited mineral media, a high medium-chain length polyhydroxyalkanoate content of 61% of cell dry weight in microbial cells was reached. The polymer consisted of 3- hydroxyhexanoate (27.2% mol) and 3-hydroxyoctanoate (72.8% mol) monomer units. Therefore, this work demonstrates a possible interconnection of feather biodegradation with keratinase and medium-chain length polyhydroxyalkanoate production.
Conflict of interest: The authors declare no conflict of interest.
- ▪ Waste feathers ▪ Pseudomonas putida ▪ keratinase ▪ mcl-PHA
How to Cite
References
Taskin M, Ozkan B, Atici O, Aydogan MN. Utilization of chicken feather hydrolysate as a novel fermentation substrate for production of exopolysaccharide and mycelial biomass from edible mushroom Morchella esculenta. Int J Food Sci Nutr. 2011; 63(5):597-602.
Stiborova H, Branska B, Vesela T, Lovecka P, Stranska M, Hajslova J, et al. Transformation of raw feather waste into digestible peptides and amino acids. J Chem Technol Biotl. 2016;91(6):1629-1637.
Poole AJ, Church JS, Huson MG. Environmentally Sustainable Fibers from Regenerated Protein. Biomacromolecules. 2009;10(1):1-8.
Keratinases. In: Current developments in biotechnology and bioengineering: production, isolation and purification of industrial products. Amsterdam: Elsevier; 2017. pp. 447-469.
Williams CM, Richter CS, Mackenzie, JR. JM, Shih Jasonch. Isolation, Identification, and Characterization of a Feather-Degrading Bacterium. Appl Environ Microb. 1990; 56 (6):1509-1515.
Mohamad N, Phang L-yee, Abd-Aziz S. Optimization of metallo-keratinase production by Pseudomonas sp. LM19 as a potential enzyme for feather waste conversion. Biocatal Biotransfor. 2017; 35(1):41-50.
Sharma R, Gupta R. Substrate specificity characterization of a thermostable keratinase from Pseudomonas aeruginosa KS-1. J Ind Microbiol Biotechnol. 2010;37:785–792.
Koller M, Maršálek L, de Sousa Dias MM, Braunegg G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N Biotechnol. 2017;37:24-38.
Follonier S. Pilot-scale Production of Functionalized mcl-PHA from Grape Pomace Supplemented with Fatty Acids. Chem. Biochem. Eng. Q. 2015;29(2):113–121.
Borrero-de Acuña J, Bielecka A, Häussler S, Schobert M, Jahn M, Wittmann C, et al. Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb Cell Fact. 2014;13(1):88-.
Escapa IF, del Cerro C, García JL, Prieto MA. The role of GlpR repressor in Pseudomonas putida KT2440 growth and PHA production from glycerol. Environ Microbiol. 2013;15(1):93-110.
Martins dos Santos VAP, Timmis KN, Tümmler B, Weinel C. Genomic Features of Pseudomonas putida Strain KT2440. In: Pseudomonas. Boston, MA: Springer US; 2004. pp. 77-112.
Nikel PI, Chavarría M, Danchin A, de Lorenzo V. From dirt to industrial applications: Pseudomonas putida as a Synthetic Biology chassis for hosting harsh biochemical reactions. Curr Opin Chem Biol. 2016;34:20-29.
Letourneau, Soussotte, Bressollier, Branland, Verneuil. Keratinolytic activity of Streptomyces sp. S.K1-02: a new isolated strain. Lett Appl Microbiol .1998;26(1):77-80.
Obruca S, Snajdar O, Svoboda Z, Marova I. Application of random mutagenesis to enhance the production of polyhydroxyalkanoates by Cupriavidus necator H16 on waste frying oil. World J Microbiol Biotechnol. 2013;29(12):2417-2428.
Taskin M, Esim N, Ortucu S. Efficient production of l-lactic acid from chicken feather protein hydrolysate and sugar beet molasses by the newly isolated Rhizopus oryzae TS-61. Food Bioprod Process. 2012 ;90(4):773-779.
Patinvoh RJ, Feuk-Lagerstedt E, Lundin M, Sárvári Horváth I, Taherzadeh MJ. Biological Pretreatment of Chicken Feather and Biogas Production from Total Broth. Appl Biochem Biotechnol. 2016 ;180(7):1401-1415.
Taskin M, Ozkan B, Atici O, Aydogan MN. Utilization of chicken feather hydrolysate as a novel fermentation substrate for production of exopolysaccharide and mycelial biomass from edible mushroom Morchella esculenta. Int J Food Sci Nutr. 2011;63(5):597-602.
Prakash S, Veeranagouda Y, Kyoung L, Sreeramulu K. Xylanase production using inexpensive agricultural wastes and its partial characterization from a halophilic Chromohalobacter sp. TPSV 101. World J Microbiol Biotechnol. 2009;25(2):197-204.
Benesova P, Kucera D, Marova I, Obruca S. Chicken feather hydrolysate as an inexpensive complex nitrogen source for PHA production by Cupriavidus necator on waste frying oils. Lett Appl Microbiol. 2017;65(2):182-188.
Obruca S, Sedlacek P, Koller M, Kucera D, Pernicova I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol Adv. 2017; in press.
Swetlana N, Jain PC. Feather degradation by strains of Bacillus isolated from decomposing feathers. Braz J Microbiol. 2010 ;41(1):196-200.
Suttiniyom C, Yammuen-art S, Kanpiengjai A, Unban K, Khanongnuch C. Digestibility and Protein Content Improvement of Corncob Silage Using Chicken Feather Partially Digested by Bacillus subtilis G8. Int J Agric Biol. 2015;17(06):1207-1212.
Gupta R, Ramnani P. Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biot. 2006;70(1):21-33.
Jacquel N, Lo C-W, Wei Y-H, Wu H-S, Wang SS. Isolation and purification of bacterial poly(3-hydroxyalkanoates). Biochem Eng J. 2008;39(1):15-27.
- Abstract Viewed: 1156 times
- PDF Downloaded: 1296 times