Expression of the Mouse HSP27 Chaperone in CHO-K1 Cells for the Enhancement of Viable Cell Density in Batch Culture Mouse HSP27-expressing CHO cells
Trends in Peptide and Protein Sciences,
Vol. 7 (2022),
7 March 2022
,
Page 1-5 (e2)
https://doi.org/10.22037/tpps.v7i.37418
Abstract
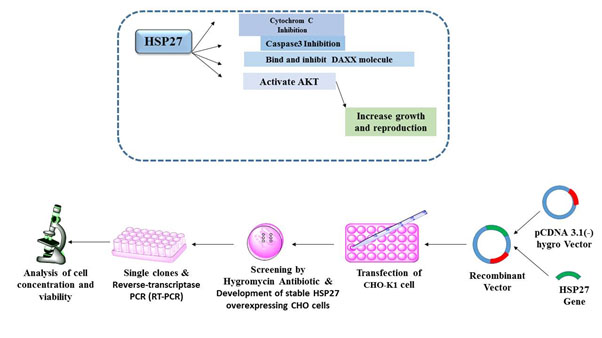
Chinese hamster ovary (CHO) cells are extremely vulnerable to cell viability loss in culture despite the availability of different nutrients supplementation strategies. As a result, extending the culture lifetime can profoundly increase recombinant protein expression. Overexpression of HSP27 and its anti-apoptotic effects have been shown in human cell lines in previous studies. In the current study, mouse HSP27 (mHSP27) was cloned in pcDNA 3.1 hygro expression vector and was expressed in CHO-K1 cells to assess its impacts on cell viability and growth. Expression of mHSP27 in CHO-K1 cells was confirmed using RT-PCR. A 3-fold enhancement in peak viable cell density of mHSP27 transfected clones was observed, and culture viability loss was delayed by 2 days compared to un-transfected cells. In future studies, the resulting mHSP27 CHO-K1 cells could be employed as a novel host system for the transient and stable expression of therapeutic recombinant proteins.
HIGHLIGHTS
- Cell engineering is an effective strategy to cope with apoptosis in CHO cells.
- HSP27 is involved in mammalian cell apoptosis.
- Expression of the mouse HSP27 increased the viability and cell density of CHO-K1 cells.
- Chinese hamster ovary cells
- Cell engineering
- Mouse HSP27
- Viability
How to Cite
References
Bruey, J.-M., C. Ducasse, P. Bonniaud, L. Ravagnan, S. A. Susin, C. Diaz-Latoud, S. Gurbuxani, A.-P. Arrigo, G. Kroemer and E. Solary (2000). "Hsp27 negatively regulates cell death by interacting with cytochrome c." Nature cell biology 2(9): 645-652.
Concannon, C., A. Gorman and A. Samali (2003). "On the role of Hsp27 in regulating apoptosis." Apoptosis 8(1): 61-70.
Coss, R., A. Sedar, S. Sistrun, C. Storck, P. Wang and P. Wachsberger (2002). "Hsp27 protects the cytoskeleton and nucleus from the effects of 42 C at pH 6.7 in CHO cells adapted to growth at pH 6.7." International journal of hyperthermia 18(3): 216-232.
Garrido, C., C. Paul, R. Seigneuric and H. Kampinga (2012). "The small heat shock proteins family: the long forgotten chaperones." The international journal of biochemistry & cell biology 44(10): 1588-1592.
Grilo, A. L. and A. Mantalaris (2019). "The increasingly human and profitable monoclonal antibody market." Trends in biotechnology 37(1): 9-16.
Ha, T. K., M. K. Jeon, D. Y. Yu and G. M. Lee (2013). "Effect of Bcl-x(L) overexpression on lactate metabolism in chinese hamster ovary cells producing antibody." Biotechnology Progress 29(6): 1594-1598.
Hwang, S. O. and G. M. Lee (2009). "Effect of Akt overexpression on programmed cell death in antibody-producing Chinese hamster ovary cells." Journal of Biotechnology 139(1): 89-94.
Jeon, M. K., D. Y. Yu and G. M. Lee (2011). "Combinatorial engineering of ldh-a and bcl-2 for reducing lactate production and improving cell growth in dihydrofolate reductase-deficient Chinese hamster ovary cells." Applied Microbiology and Biotechnology 92(4): 779-790.
Kim, Y. G., J. Y. Kim and G. M. Lee (2009). "Effect of XIAP overexpression on sodium butyrate-induced apoptosis in recombinant Chinese hamster ovary cells producing erythropoietin." Journal of Biotechnology 144(4): 299-303.
Lalonde, M.-E. and Y. Durocher (2017). "Therapeutic glycoprotein production in mammalian cells." Journal of biotechnology 251: 128-140.
Lanneau, D., M. Brunet, E. Frisan, E. Solary, M. Fontenay and C. Garrido (2008). "Heat shock proteins: essential proteins for apoptosis regulation." Journal of cellular and molecular medicine 12(3): 743-761.
Lee, Y. Y., K. T. Wong, J. Tan, P. C. Toh, Y. Mao, V. Brusic and M. G. Yap (2009). "Overexpression of heat shock proteins (HSPs) in CHO cells for extended culture viability and improved recombinant protein production." Journal of Biotechnology 143(1): 34-43.
Misaghi, S., Y. Qu, A. Snowden, J. Chang and B. Snedecor (2013). "Resilient immortals, characterizing and utilizing Bax/Bak deficient Chinese hamster ovary (CHO) cells for high titer antibody production." Biotechnology Progress 29(3): 727-737.
Pandey, P., R. Farber, A. Nakazawa, S. Kumar, A. Bharti, C. Nalin, R. Weichselbaum, D. Kufe and S. Kharbanda (2000). "Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3." Oncogene 19(16): 1975-1981.
Sung, Y. H., J. S. Lee, S. H. Park, J. Koo and G. M. Lee (2007). "Influence of co-down-regulation of caspase-3 and caspase-7 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin." Metabolic Engineering 9(5-6): 452-464.
Tan, J. G., Y. Y. Lee, T. Wang, M. G. Yap, T. W. Tan and S. K. Ng (2015). "Heat shock protein 27 overexpression in CHO cells modulates apoptosis pathways and delays activation of caspases to improve recombinant monoclonal antibody titre in fed batch bioreactors." Biotechnology journal 10(5): 790-800.
Vives, J., S. Juanola, J. J. Cairó and F. Gòdia (2003). "Metabolic engineering of apoptosis in cultured animal cells: implications for the biotechnology industry." Metabolic engineering 5(2): 124-132.
Walsh, G. (2018). "Biopharmaceutical benchmarks 2018." Nature biotechnology 36(12): 1136-1145.
- Abstract Viewed: 273 times
- PDF Downloaded: 190 times