Lactic Acid Bacteria Producing Sorbic Acid and Benzoic Acid Compounds from Fermented Durian Flesh (Tempoyak) and Their Antibacterial Activities Against Foodborne Pathogenic Bacteria
Applied Food Biotechnology,
Vol. 8 No. 2 (2021),
16 March 2021
,
Page 121-132
https://doi.org/10.22037/afb.v8i2.32749
Abstract
Background and Objective:
Antibacterial compounds produced by lactic acid bacteria are believed to replace functions of chemical preservatives. The objectives of this study were to identify lactic acid bacteria, which produced antibacterial compounds, from fermented durian flesh (tempoyak) and to assess antibacterial activities of the isolates.
Material and Methods:
Two bacterial identification techniques were used, including API 50 CHL kit with supplementary medium and matrix-assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF/MS).
Results and Conclusion:
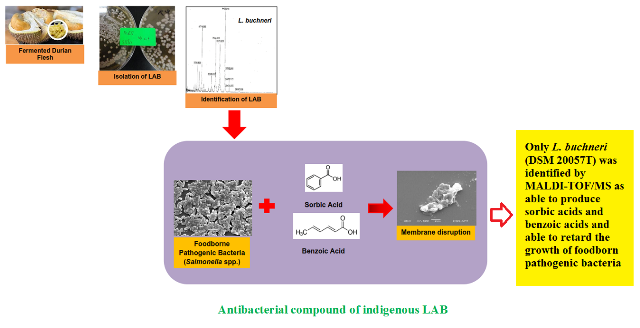
Four various lactic acid bacteria strains of Lactobacillus buchneri, Lactobacillus plantarum, Lactobacillus brevis I and Lactobacillus acidophilus I were identified using API 50 CHL Kit and five various others of Lactobacillus paracasei DSM 2649, Lactobacillus buchneri DSM 20057T, Lactobacillus parabuchneri DSM 57069, Lactobacillus paracasei DSM 20020 and Lactobacillus farcimini CIP 103136T using MALDI-TOF/MS. Cell-free supernatant extracted from Lactobacillus plantarum, Lactobacillus buchneri, Lactobacillus brevis I and Lactobacillus acidophilus I included strong inhibitory effects against Vibrio cholera O1 (Inaba type), Vibrio cholera O139 (Bengal type), Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 11795, Escherichia coli O157, Salmonella typhimurium ATCC 14028 and a total of 23 serotypes of Salmonella spp. associated with outbreaks of food poisoning from raw chicken, egg shell and water samples. Only Lactobacillus buchneri DSM 20057T was identified by MALDI-TOF/MS as a strain producing sorbic and benzoic acids. This strain can potentially be used as food preservative to decrease growth of foodborne pathogenic bacteria.
- ▪ Antibacterial Activity
- ▪ Cell Free Supernatant
- ▪ Chemical Preservatives
- ▪ Lactic Acid Bacteria
- ▪ Tempoyak (Durian Flesh)
How to Cite
References
Rather IA, Koh WY, Paek WK, Lim J. The sources of chemical contaminants in food and their health implications. Front Pharmacol. 2017; 8: 1-8.
doi:10.3389/fphar.2017.00830.
Garvey M. Food pollution: A comprehensive review of chemical and biological sources of food contamination and impact on human health. Nutr. 2019; 44(1).
doi:10.1186/s41110-019-0096-3.
Batz MB, Richardson LC, Bazaco MC, Parker C, Chirtel SJ, Cole D, Golden NJ, Griffin PM, Gu W, Schmitt SK, Wolpert BJ, Kufel JSZ, Hoekstra RM. Recency-weighted statistical modeling approach to attribute illnesses caused by 4 pathogens to food sources using outbreak data, United States. Emerg Infect Dis. 2021; 27(1): 214-222.
doi:10.3201/eid2701.203832
Tack DM, Marder EP, Griffin PM, Cieslak PR, Dunn J, Hurd S, Scallan E, Lathrop S, Muse A, Ryan P, Smith K, Tobin-D’Angelo M, Vugia DJ, Holt KG, Wolpert BJ, Tauxe R, Geissler AL. Preliminary incidence and trends of infections with pathogens transmitted commonly through food-Foodborne diseases active surveillance network, 10 U.S. sites, 2015-2018. Morbidity and Mortality Weekly Report (MMWR),available from:
https://www.cdc.gov/mmwr/volumes/68/wr/pdfs/mm6816a2-H.pdf [Accessed 26 April 2006]
New CY, Ubong A, Premarathne, JMKJK, Thung TY, Lee E, Chang WS, Loo YY, Kwan SY, Tan CW, Kuan CH, Son R. Microbiological food safety in Malaysia from the academician’s perspective. Food Res. 2017; 1(6): 183-202.
Salleh F, Lani MN, Wan-Abdullah WZ, Tuan-Chilek TZ, Hassan Z. A review on incidences of foodborne diseases and interventions for a better national food safety system in Malaysia. Malaysian Appl Biol. 2017; 46(3): 1-7.
Hashempour-Baltork F, Hosseini H, Shojaee-Aliabadi S, Torbati M, Alizadeh AM, Alizadeh M. Drug resistance and the prevention strategies in food borne bacteria: An update review. Adv Pharma Bull. 2019; 9(3): 335-347.
doi: 10.15171/apb.2019.041
Juliyarsi I, Hartini P, Yuherman DA, Arief, Purwanto H, Aritonang SN, Hellyward J, Purwati E. Characterisation of lactic acid bacteria and determination of antimicrobial activity in tempoyak from Padang Pariaman district, West Sumatra, Indonesia. Pakistan J Nutr. 2018; 17: 506-511.
doi:10.3923/pjn.2018.506.511
Wasnin RM, Abdul Karim MS, Mohd Ghazali H. Effect of temperature-controlled fermentation on physicochemical properties and lactic acid bacterial count of durian (Durio zibethinus Murr.) pulp. J Food Sci Technol. 2014; 51: 2977-2989.
doi:10.1007/s13197-012-0869-7
Amin AM, Jaafar Z, Khim LN. Effect of salt on Tempoyak fermentation and sensory evaluation. J Biol Sci. 2004; 4: 650-653.
doi: 10.3923/jbs.2004.650.653
Leisner JJ, Vancanneyt M, Rusul G, Pot B, Lefebvre K, Fresi A, Tee LK. Identification of lactic acid bacteria constituting the predominating microflora in an acid-fermented condiment (Tempoyak) popular in Malaysia. Int J Food Microbiol. 2001; 63(1-2): 149-157.
doi: 10.1016/s0168-1605(00)00476-1
Chuah LO, Shamila-Syuhada AK, Liong MT, Rosma A, Thong KL, Rusul G. Physicochemical, microbiological properties of Tempoyak and molecular characterization of lactic acid bacteria isolated from Tempoyak. Food Microbiol. 2016; 58: 95-104.
doi:10.1016/j.fm.2016.04.002
Salleh F, Lani MN, Ismail N. Antimicrobial activity of cell-free supernatant of lactic acid bacteria isolated from fermented durian flesh against multiple antibiotic resistances, Salmonella associated with food poisoning cases in Malaysia. IOSR J Pharm Biol Sci. 2014; 9(6): 60-65.
doi: 10.9790/3008-09646065
Ahmad A, Yap WB, Kofli NT, Ghazali AR. Probiotic potentials of Lactobacillus plantarum isolated from fermented durian (Tempoyak), a Malaysian traditional condiment. Food Sci Nutr. 2018; 6: 1370-1377.
doi:10.1002/fsn3.672
Veron HE, Risio HDD, Isla MI, Torres S. Isolation and selection of potential probiotic lactic acid bacteria from Opuntia ficus-indica fruits that grow in Northwest Argentina. LWT- Food Sci Technol. 2017; 84: 231-240.
doi:10.1016/j.lwt.2017.05.058
Nuraida L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. FSHW. 2015; 4: 47-55.
doi: 10.1016/j.fshw.2015.06.001.
Yuliana N, Dizon EI. Phenotypic identification of lactic acid bacteria isolated from Tempoyak (Fermented durian) made in the Philippines. Int J Biol. 2011; 3(2): 145-152.
doi:10.5539/ijb.v3n2p145
Muhialdin BJ, Hassan Z, Ahmed Imdakim MM, Abdul Kahar FKS, Mustafa AM. Malaysian isolates of lactic acid bacteria with antibacterial activity against Gram-positive and Gram-negative pathogenic bacteria. J Food Res. 2012; 1(1): 110-116. doi:10.5539/jfr. v1n1p110
Mohd Adnan AF, Tan IKP. Isolation of lactic acid bacteria from Malaysian foods and assessment of the isolates for industrial potential. Bioresour Technol. 2007; 98: 1380-1385.
doi: 10.1016/j.biortech.2006.05.034
Issa ZM. Molecular characterisation of Lactobacillus plantarum Isolated from Malaysian Fermented Foods. Master‘s Dissertation; Universiti Putra Malaysia, Malaysia. 2000; pp. 134.
Wirawati CU. Potensi bakeri asam laktat yang diisolasi dari Tempoyak sebagai probiotik. Master of Science‘s Dissertation; Bogor, Institut Pertanian Bogor, Indonesia. 2002; pp.75.
Ekowati CN. Mikroflora pada fermentasi daging buah durian (tempoyak). J Sci Technol. 1998; 30(4): 136-141.
de Lacerda JRM, da Silva TF, Vollu RE, Marques, JM, Seldin L. Generally recognized as safe (GRAS) Lactococcus lactis strains associated with Lippia sidoides Cham. Are able to solubilize / mineralize phosphate. SpringerPlus, 2016; 5(1): 828.
doi: 10.1186/s40064-016-2596-4
Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Env Microbiol. 1999; 65(9): 3763-3766. doi: 10.1128/AEM.65.9.3763-3766.1999
Ahmad A, Yap WB, Kofli NT, Ghazali AR. Probiotic potentials of Lactobacillus plantarum isolated from fermented durian (Tempoyak), a Malaysian traditional condiment. Food Sci. Nutr. 2018; 6(6): 1370-1377.
doi: 10.1002/fsn3.672
Khalil ES, Abd Manap MY, Mustafa S, Alhelli AM, Shokryazdan P. Probiotic properties of exopolysaccharides-producing Lactobacillus strains isolated from Tempoyak. Molecules 2017; 23(2): 398.
doi: 10.3390/molecules23020398
Callaway A, Kostrzewa M, Willershausen B, Schmidt F, Thiede B, Kȕpper H, Kneist S. Identification of Lactobacilli from deep carious lesions by means of species-specific PCR and MALDI-TOF mass spectrometry. Clin Lab. 2013; 59(11-12): 1373-1379.
doi:10.7754/clin.lab. 2013.121225
Perez C, Paul M, Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990; 15:113-115.
Brown PN, Chan M, Paley L, Betz JM. Determination of major phenolic compounds in Echinacea spp. raw materials and finished products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation matrix extension. J AOAC Int. 2011; 94(5): 1400-1410.
doi: 10.5740/jaoacint.11142
Oxoid Manual. Dehydrated culture media. MRS Agar (De Man, Rogosa and Sharpe).Available from: http://www.-oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0361&c=UK&lang=EN
[Accessed 30June 2020]
Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S, Walker B. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriogenic lactic acid bacteria. AMB Exp. 2018; 8(10): 1-14.
doi: 10.1186/s13568-018-0536-0
LeBlanc JG, Garro MS, de Giori SG. Effect of pH on Lactobacillus fermentum growth, raffinose removal, α-galactosidase activity and fermentation products. Appl Microbiol Biotechnol. 2014; 65(1):119-123.
doi: 10.1007/s00253-003-1532-z
Mataragas M, Metaxopoulos J, Galiotou M, Drosinos EH. Influence of pH and temperature by Leuconostoc mesenteroides L124 and Lactobacillus curvatus L442. Meat Sci. 2003; 64(3): 265-271.
doi: 10.1016/S0309-1740(02)00188-2
Boyd MA, Antonio MAD, Hillier SL. Comparison of API 50 CH strips to whole-chromosomal DNA probes for identification of Lactobacillus species. J Clin Microbiol. 2005; 43 (10): 5309-5311.
doi: 10.1128/JCM.43.10.5309-5311.2005
Elmacı SB, Tokatlı M, Dursun D, Ozçelik F, Şanlıbaba P. Phenotypic and genotypic identification of lactic acid bacteria isolated from traditional pickles of the Çubuk region in Turkey. Folia Microbiol. 2015; 60: 241-251.
doi: 10.1007/s12223-014-0363-x
Reuben RC, Roy PC, Sarkar SL, Alam RU, Jahid IK. Isolation, characterization and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019; 19: 253.
doi: 10.1186/s12866-019-1626-0
Ni K, Wang Y, Li D, Cai Y, Pang H. Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. Plos One. 2015;
(3): e0121967.
doi: 10.1371/journal.pone.0121967.
Goswami G, Bora SS, Parveen A, Boro RC, Barooah M. Identification and functional properties of dominant lactic acid bacteria isolated from Kahudi, a traditional rapeseed fermented food product of Assam, India. J Ethnic Food. 2017; 4(3):187-197.
doi: 10.1016/j.jef.2017.08.008
Ketsa S, Daengkanit T. Physiological changes during postharvest ripening of durian fruit (Durio zibethinus Murray). J Horticult Sci Biotechnol. 1998; 73: 575-577.
doi:10.1080/14620316.1998.11511017
Leisner JJ, Vancanneyt M, Lefebvre K, Vandemeulebroecke K, Hoste B, Vilalta NE, Rusul G, Swings J. Lactobacillus durianis sp. nov., isolated from an acid-fermented condiment (tempoyak) in Malaysia. Int J Syst Evol Microbiol. 2002; 52: 927-931.
doi:10.1099/00207713-52-3-927
Salomskiene J, Jonkuviene D, Macioniene I, Abraitiene A, Zeime J, Repeckiene J, Vaiciulyte Funk J. Differences in the occurrence and efficiency of antimicrobial compounds produ-ced by lactic acid bacteria. Eur Food Res Technol. 2019; 245(3): 1-11.
doi:10.1007/s00217-018-03227-3
Amrutha B, Sundar K, Shetty PH. Effect of organic acids on biofilm formation and quorum signalling of pathogens from fresh fruits and vegetables. Microb Pathog. 2017; 111: 156-162.
doi: 10.1016/j.micpath.2017.08.042
Halstead FD, Rauf M, Moiemen NS, Bamford A, Wearn CM, Fraise AP, Lund PA, Oppenheim BA, Webber MA. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. Plos One. 2015; 10(9): e0136190.
doi: 10.1371/journal.pone.0136190
Sullivan DJ, Azlin-Hasim S, Cruz-Romero M, Cummins E, Kerry JP, Morris MA. Natural Antimicrobial Materials for Use in Food Packaging, In A. Tiwari (edition.). Handbook of Antimicrobial Coatings. Elsevier. 2018: pp. 181-233.
Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res Int. 2018; 9361614: 1-18. doi:10.1155/2018/9361614.
oMed Res Int. 2018; 9361614: 1-18. doi:10.1155/2018/9361614
- Abstract Viewed: 1475 times
- pdf Downloaded: 982 times
