Optimization of Protease Production by Psychrotrophic Rheinheimera sp. with Response Surface Methodology
Applied Food Biotechnology,
Vol. 3 No. 4 (2016),
1 October 2016
,
Page 236-245
https://doi.org/10.22037/afb.v3i4.12776
Abstract
Background and Objectives: Psychrotrophic bacteria can produce enzymes at low temperatures; this provides a wide biotechnological potential, and offers numerous economical advantages over the use of mesophilic bacteria. In this study, extracellular protease production by psychrotrophic Rheinheimera sp. (KM459533) was optimized by the response surface methodology.
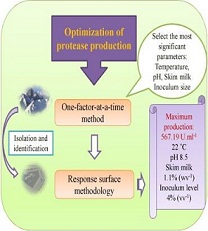
Materials and Methods: The culture medium was tryptic soy broth containing 1% (w v -1 ) skim milk. First, the effects of variables were independently evaluated on the microbial growth and protease production by one-factor-at-a-time method within the following ranges: incubation time 24-120 h, temperature 15-37°C, pH 6- 11, skim milk concentration 0-2% (w v -1 ), and inoculum size 0.5-3% (v v -1 ). The combinational effects of the four major variable including temperature, pH, skim milk concentration, and inoculum size were then evaluated within 96 h using response surface methodology through 27 experiments.
Results and Conclusion: In one-factor-at-a-time method, high cell density was detected at 72h, 20°C, pH 7, skim milk 2% (w v -1 ), and inoculum size 3% (v v -1 ), and maximum enzyme production (533.74 Uml-1 ) was achieved at 96h, 20°C, pH 9, skim milk 1% (w v -1 ), and inoculum size 3% (v v -1 ). The response surface methodology study showed that pH is the most effective factor in enzyme production, and among the other variables, only temperature had significant interaction with pH and inoculum size. The determination coefficient (R2 =0.9544) and non-significant lack of fit demonstrated correlation between the experimental and predicted values. The optimal conditions predicted by the response surface methodology for protease production were defined as: 22C, pH 8.5, skim milk 1.1% (w v -1 ), and inoculum size 4% (v v -1 ). Protease production under these conditions reached to 567.19 Uml-1 . The use of response surface methodology in this study increased protease production by eight times as compared to the observed before optimization.
Conflict of interests: The authors declare no conflict of interest.
- Cold-tolerant
- Optimization
- Protease production
- Rheinheimera sp.
How to Cite
References
Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev. 1998;62(3):597-635. doi: 1092-2172/98/04.00.
Sharma J, Mathur N, Singh A. Protease Production from Polyextremophilic Bacteria. Int J Curr Microbiol App Sci. 2016;5(5):807-815. doi: 10.20546/ijcmas-.2016.505.081.
Siddiqui KS. Some like it hot, some like it cold: Temperature dependent biotechnological applications and improvements in extremophilic enzymes. Biotechnol Adv. 2015;33(8):1912-1922. doi: 10.1016-/j.biotechadv.2015.11.001.
Puri S, Beg QK, Gupta R. Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr Microbiol. 2002;44:286-90. doi: 10.1007/s00284-001-0006-8.
Singh SK, Singh SK, Tripathi VR, Khare SK, Garg SK. Comparative one-factor-at-a-time, response surface (statistical) and bench-scale bioreactor level optimization of thermoalkaline protease production from a psychrotrophic Pseudomonas putida SKG-1 isolate. Microb Cell Fact. 2011;10:114-127. doi: 10.1186/1475-2859-10-114.
Wang Q, Hou Y, Xu Z, Miao J, Li G. Optimization of cold-active protease production by the psychrophilic bacterium Colwellia sp. NJ341 with response surface methodology. Bioresour Technol. 2008;99(6):1926-1931. doi: 10.1016/j.biortech.2007.03.028.
Dutta JR, Dutta PK, Banerjee R. Optimization of culture parameters for extracellular protease production from a newly isolated Pseudomonas sp. using response surface and artificial neural network models. Process Biochem. 2004;39(12):2193-2198. doi: 10.1016/j.procbio.2003.11.009.
Kuddus M, Ramteke PW. Purification and Properties of Cold-active Metalloprotease from Curtobacterium luteum and Effect of Culture Conditions on Production. Chin J Biotechnol. 2008;24(12):2074-2080. doi: 10.1016/S1872-2075(09)60012-1.
da Silva Nascimento TCE, de Sena AR, Gomes JEG, dos Santos WL, Montalvo GSA, Tambourgi EB, et al. Extracellular serine proteases by Acremonium sp. L1-4B isolated from Antarctica: Overproduction using cactus pear extract with response surface methodology. Biocatal Agric Biotechnol. 2015;4(4):737-744. doi: 10.1016/j.bcab.2015.10.006.
Lee J, Jung Y-J, Lee HK, Hong SG, Kim O-S. Complete genome sequence of Pedobacter cryoconitis PAMC 27485, a CRISPR-Cas system-containing psychrophile isolated from Antarctica. J Biotechnol. 2016;226:74-75. doi: j.jbiotec.2016.03.035.
Salwan R, Kasana RC. Purification and characterization of an extracellular low temperature-active and alkaline stable peptidase from psychrotrophic Acinetobacter sp. MN 12 MTCC (10786). Indian J Microbiol. 2013;53(1):63-69. doi: 10.1007/s12088-012-0344-1.
Tari C, Genckal H, Tokatlı F. Optimization of a growth medium using a statistical approach for the production of an alkaline protease from a newly isolated Bacillus sp. L21. Process Biochem. 2006;41(3):659-665. doi: 10.1016/j.procbio.200-5.08.012.
Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK. Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub‐glacial lakes of NW Indian Himalayas. J Basic Microbiol. 2016;56:294-307. doi: 10.1002/jobm.201500230.
Yang B, Wang Y, Qian P-Y. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinformatics. 2016;17(1):135-143. doi: 10.1186/s12859-016-0992-y.
Han SJ, Park H, Kim S, Kim D, Park HJ, Yim JH. Enhanced Production of Protease by Pseudoalteromonas Arctica PAMC 21717 via Statistical Optimization of Mineral Components and FED-Batch Fermentation. Prep Biochem Biotechnol. 2015;46(4):328-335. doI: 10.1080/10826068.2015.10-31390.
Kunitz M. Crystalline soybean trypsin inhibitor II. General properties. J Gen Physiol. 1947;30(4):291-310. doi: 10.1085/jgp.30.4.291.
Unver Y, Yildiz M, Taskin M, Arslan NP, Ortucu S. Protease production by free and immobilized cells of the cold-adapted yeast Cryptococcus victoriae CA-8. Biocatal Biotransformation. 2015;33(2):105-110. doi: 10.3109/10242422.2015.1060229.
McFarland J. The nephelometer: an instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA. 1907;49(14):1176-1178. doi: 10.1001/jam-a.1907.25320140022001f.
Maran JP, Manikandan S, Priya B, Gurumoorthi P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. JFST. 2015;52:92-104. doi: 10.1007/s13197-013-0985-z.
Morita RY. Psychrophilic bacteria. Bacteriol Rev. 1975;39:144. doi: 10.1016/j.aca.2007.07.011.
Kuddus M, Ramteke PW. Cold-active extracellular alkaline protease from an alkaliphilic Stenotrophomonas maltophilia: production of enzyme and its industrial applications. Can J Microbiol. 2009;55:1294-301. doi: 10.1139/W09-089.
Sinsuwan S, Jangchud A, Rodtong S, Roytrakul S, Yongsawatdigul J. Statistical optimization of the production of NaCl-tolerant proteases by a moderate halophile, Virgibacillus sp. SK37. Food Technol. Biotechnol. 2015;53:136-45. doi: 10.17113/ft b.53.02.15.4015.
Dube S, Singh L, Alam S. Proteolytic anaerobic bacteria from lake sediments of Antarctica. Enzyme Microb Technol. 2001;28(1):114-21. doi: 10.1016/S0141-0229(00)00287-8.
- Abstract Viewed: 1160 times
- Full article Downloaded: 877 times

