Cytoplasmic Expression of Human Bone Morphogenetic Protein-7 by a Genetically Engineered Strain of Escherichia coli, SHuffle® Strain Human BMP-7 soluble expression by SHuffle® strain
Trends in Peptide and Protein Sciences,
Vol. 7 (2022),
7 March 2022
,
Page 1-7 (e7)
https://doi.org/10.22037/tpps.v7i.39039
Abstract
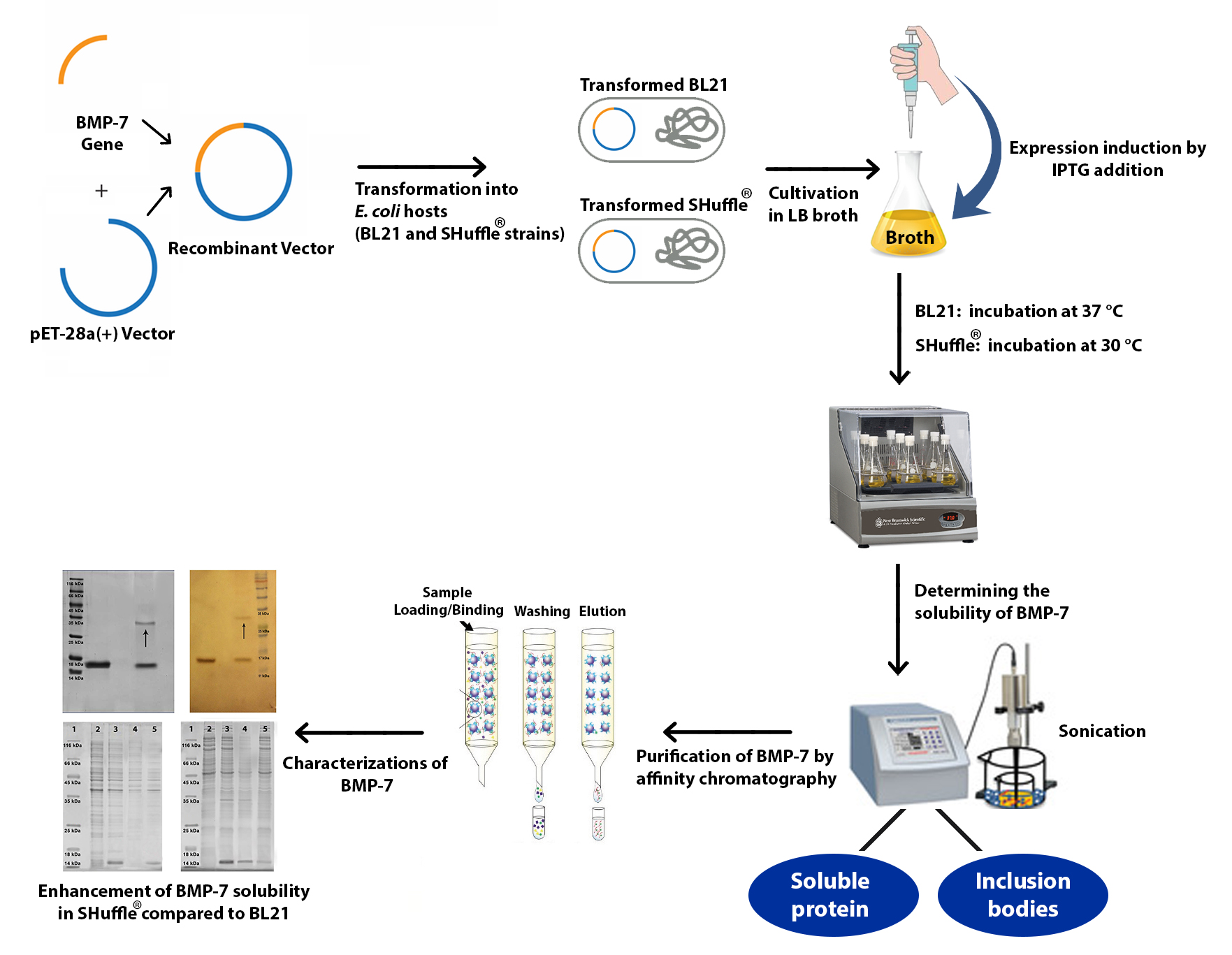
Homodimeric bone morphogenetic protein-7 (BMP-7) plays a key role in bone metabolism. The functionality of human BMP-7 protein is dependent on its disulfide bond formation and proper folding. Therefore, the expression of soluble recombinant BMP-7 using Escherichia coli cells as the host remains a challenge. Given the need for these disulfide-bonded proteins for stabilized native conformation, the cytoplasm of SHuffle® T7 Express as an E. coli engineered strain can effectively fold disulfide-bonded proteins with a need for proper oxidative folding. These cellular features turn the SHuffle® expression system into an efficient host for the recombinant production of human BMP-7 protein. A soluble dimeric form of recombinant human BMP-7 (rhBMP-7) which has a wide range of applications in medicine and can be used in the treatment of bone defects was produced using the SHuffle® strain as the expression system. This study demonstrated the production of rhBMP-7 using E. coli SHuffle® T7 Express strain. Also, an effective protocol was proposed for the expression and purification of soluble human BMP-7. In addition, it was found that the genetically engineered SHuffle® strain can efficiently enhance the solubility of recombinant human BMP-7 as a therapeutic target.
HIGHLIGHTS
- E. coli SHuffle® T7 Express strain is an effective host to express disulfide-bonded proteins.
- BMP-7 is involved in the process of bone formation.
- Expression of human BMP-7 in SHuffle® strain increased its solubility.
- BMP-7
- Soluble expression
- Recombinant protein
- Disulfide bond
- E. coli
- SHuffle® T7 Express strain
How to Cite
References
Ahmadzadeh, M., Farshdari, F., Nematollahi, L., Behdani, M. and E. Mohit, (2020). ″Anti-HER2 scFv expression in Escherichia coli SHuffle® T7 express cells: effects on solubility and biological activity.″ Molecular Biotechnology, 62(1): 18-30. DOI: https://doi.org/10.1007/s12033-019-00221-2.
Bessa, P.C., Casal, M. and R. Reis, (2008). ″Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery).″ Journal of Tissue Engineering and Regenerative Medicine, 2(2-3): 81-96. DOI: https://doi.org/10.1002/term.74.
Bessa, P.C., Cerqueira, M., Rada, T., Gomes, M.E., Neves, N., Nobre, A., Reis, R. and M. Casal, (2009). ″Expression, purification and osteogenic bioactivity of recombinant human BMP-4,-9,-10,-11 and-14.″ Protein Expression and Purification, 63(2): 89-94. DOI: https://doi.org/10.1016/j.pep.2008.09.014.
Bessette, P. H., Åslund, F., Beckwith, J. and G. Georgiou, (1999). ″Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm.″ Proceedings of the National Academy of Sciences, 96(24): 13703-13708. DOI: https://doi.org/10.1073/pnas.96.24.13703.
Cheng, H., Jiang, W., Phillips, F.M., Haydon, R. C., Peng, Y., Zhou, L., Luu, H. H., An, N., Breyer, B. and P. Vanichakarn, (2003). ″Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). Journal of Bone and Joint Surgery JBJS, 85(8): 1544-1552. DOI: https://doi.org/10.2106/00004623-200308000-00017.
Collet, J.-F. and J. Messens, (2010). ″Structure, function, and mechanism of thioredoxin proteins.″ Antioxidants and Redox Signaling, 13(8): 1205-1216. DOI: https://doi.org/10.1089/ars.2010.3114.
Derman, A.I., Prinz, W.A., Belin, D. and J. Beckwith, (1993). ″Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli.″ Science, 262(5140): 1744-1747. DOI: https://doi.org/10.1126/science.8259521.
Devi, V.S. and P.R. Mittl, (2011). ″Monitoring the disulfide bond formation of a cysteine-rich repeat protein from Helicobacter pylori in the periplasm of Escherichia coli.″ Current Microbiology, 62(3): 903-907. DOI: https://doi.org/10.1007/s00284-010-9803-2.
Fathi-Roudsari, M., Akhavian-Tehrani, A. and N. Maghsoudi, (2016). ″Comparison of three Escherichia coli strains in recombinant production of reteplase.″ Avicenna Journal of Medical Biotechnology, 8(1): 16. PMID: 26855731.
Francis, D.M. and R. Page, (2010). ″Strategies to optimize protein expression in E. coli.″ Current Protocols in Protein Science, 61(1): 5.24.1-5.24.29. DOI: https://doi.org/10.1002/0471140864.ps0524s61.
Gopal, G.J. and A. Kumar, (2013). ″Strategies for the production of recombinant protein in Escherichia coli.″ The Protein Journal, 32(6): 419-425. DOI: https://doi.org/10.1007/s10930-013-9502-5.
Hernández, V.E.B., Maldonado, L.M.P., Rivero, E.M., De La Rosa, A.P.B., Jiménez-Bremont, J.F., Acevedo, L.G.O. and A.D.L. Rodríguez, (2008). Periplasmic expression and recovery of human interferon gamma in Escherichia coli. Protein Expression and Purification, 59(1): 169-174. DOI: https://doi.org/10.1016/j.pep.2008.01.019.
Jalomo-Khayrova, E., Mares, R.E., Muñoz, P.L., Meléndez-López, S.G., Rivero, I.A. and M.A. Ramos, (2018). ″Soluble expression of an amebic cysteine protease in the cytoplasm of Escherichia coli SHuffle Express cells and purification of active enzyme.″ BMC Biotechnology, 18(1): 1-7. DOI: https://doi.org/10.1186/s12896-018-0429-y.
Kasekarn, W., Suksiriphattanapong, B., Chokepaichitkool, T., Wanachewin, O., Roytrakul, S. and P. Kongtawelert, (2020). ″Soluble expression and purification of bioactive recombinant human bone morphogenetic protein-2 from Escherichia coli.″ Chiang Mai University Journal of Natural Sciences, 19(4): 752. DOI: https//:doi.org/10.12982/CMUJNS.2020.0048.
Kirker-Head, C.A. (2000). ″Potential applications and delivery strategies for bone morphogenetic proteins.″ Advanced Drug Delivery Reviews, 43(1): 65-92. DOI: https://doi.org/10.1016/S0169-409X(00)00078-8.
Klösch, B., Fürst, W., Kneidinger, R., Schuller, M., Rupp, B., Banerjee, A. and H. Redl, (2005). ″Expression and purification of biologically active rat bone morphogenetic protein-4 produced as inclusion bodies in recombinant Escherichia coli.″ Biotechnology Letters, 27(20): 1559-1564. DOI: https://doi.org/10.1007/s10529-005-1794-x.
Kübler, N., Würzler, K., Reuther, J., Sieber, E., Kirchner, T. and W. Sebald, (2000). ″Effect of different factors on the bone forming properties of recombinant BMPs.″ Mund-, Kiefer-und Gesichtschirurgie: MKG, 4(Suppl2): S465-9. DOI: https://doi.org/10.1007/pl00012693.
Kurokawa, Y., Yanagi, H. and T. Yura, (2001). ″Overproduction of bacterial protein disulfide isomerase (DsbC) and its modulator (DsbD) markedly enhances periplasmic production of human nerve growth factor in Escherichia coli.″ Journal of Biological Chemistry, 276(1): 14393-14399. DOI:https://doi.org/10.1074/jbc.M100132200.
Larentis, A.L., Nicolau, J.F.M.Q., Esteves, G.D.S., Vareschini, D.T., De Almeida, F.V.R., Dos Reis, M.G., Galler, R. and M.A. Medeiros, (2014). ″Evaluation of pre-induction temperature, cell growth at induction and IPTG concentration on the expression of a leptospiral protein in E. coli using shaking flasks and microbioreactor.″ BMC Research Notes, 7(1): 1-13. DOI: https://doi.org/10.1186/1756-0500-7-671.
Levy, R., Weiss, R., Chen, G., Iverson, B.L. and G. Georgiou, (2001). ″Production of correctly folded Fab antibody fragment in the cytoplasm of Escherichia coli trxB gor mutants via the coexpression of molecular chaperones.″ Protein Expression and Purification, 23(2): 338-347. DOI: https://doi.org/10.1006/prep.2001.1520.
Lobstein, J., Emrich, C.A., Jeans, C., Faulkner, M., Riggs, P. and M. Berkmen, (2012). ″SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm.″ Microbial Cell Factories, 11(1): 1-16. DOI: https://doi.org/10.1186/1475-2859-11-56.
Martínez-Alonso, M., García-Fruitós, E., Ferrer-Miralles, N., Rinas, U. and A. Villaverde, (2010). ″Side effects of chaperone gene co-expression in recombinant protein production.″ Microbial Cell Factories, 9(1): 1-6. DOI: https://doi.org/10.1186/1475-2859-9-64.
Ritthisan, P., Ojima-Kato, T., Damnjanović, J., Kojima, T. and H. Nakano, (2018). ″SKIK-zipbody-alkaline phosphatase, a novel antibody fusion protein expressed in Escherichia coli cytoplasm.″ Journal of Bioscience and Bioengineering, 126(6): 705-709. DOI: https://doi.org/10.1016/j.jbiosc.2018.06.009.
Ritz, D., Lim, J., Reynolds, C.M., Poole, L.B. and J. Beckwith, (2001). ″Conversion of a peroxiredoxin into a disulfide reductase by a triplet repeat expansion.″ Science, 294(5540): 158-160. DOI: https://doi.org/10.1126/science.1063143.
Robbens, J., De Coen, W., Fiers, W. and E. Remaut, (2006). ″Improved periplasmic production of biologically active murine interleukin-2 in Escherichia coli through a single amino acid change at the cleavage site.″ Process Biochemistry, 41(6): 1343-1346. DOI: https://doi.org/10.1016/j.procbio.2006.01.009.
Safarpour, H., Banadkoki, S.B., Keshavarzi, Z., Morowvat, M.H., Soleimanpour, M., Pourmolaei, S. and F.H. Shirazi, (2017). ″Expression analysis and ATR-FTIR characterization of the secondary structure of recombinant human TNF-α from Escherichia coli SHuffle® T7 Express and BL21 (DE3) cells.″ International Journal of Biological Macromolecules, 99: 173-178. DOI: https://doi.org/10.1016/j.ijbiomac.2017.02.052.
Soares, C.R., Gomide, F.I., Ueda, E.K. and P. Bartolini, (2003). ″Periplasmic expression of human growth hormone via plasmid vectors containing the λPL promoter: use of HPLC for product quantification.″ Protein Engineering, 16(12): 1131-1138. DOI: https://doi.org/10.1093/protein/gzg114.
Tóth, F., Tőzsér, J. and C. Hegedűs, (2021). ″Effect of Inducible BMP-7 Expression on the Osteogenic Differentiation of Human Dental Pulp Stem Cells.″ International Journal of Molecular Sciences, 22(12): 6182. DOI: https://doi.org/10.3390/ijms22126182.
Urist, M.R. and B.S. Strates, (1971). ″Bone morphogenetic protein.″ Journal of Dental Research, 50(6): 1392-1406. DOI: https://doi.org/10.1177/00220345710500060601.
Westerhuis, R., Van Bezooijen, R. and P. Kloen, (2005). ″Use of bone morphogenetic proteins in traumatology.″ Injury, 36(12): 1405-1412. DOI: https://doi.org/10.1016/j.injury.2005.02.047.
Zhang, H., Wu, J., Zhang, Y., Fu, N., Wang, J. and S. Zhao, (2010). ″Optimized procedure for expression and renaturation of recombinant human bone morphogenetic protein-2 at high protein concentrations.″ Molecular Biology Reports, 37(7): 3089-3095. DOI: https://doi.org/10.1007/s11033-009-9883-x.
- Abstract Viewed: 226 times
- PDF Downloaded: 158 times