Methylglyoxal Binding to Bovine Liver Catalase Results in Loss of Activity and Heme Dislocation
Trends in Peptide and Protein Sciences,
Vol. 4 (2019),
1 Dey 2019
,
Page 1-8 (e6)
https://doi.org/10.22037/tpps.v4i0.26261
Abstract
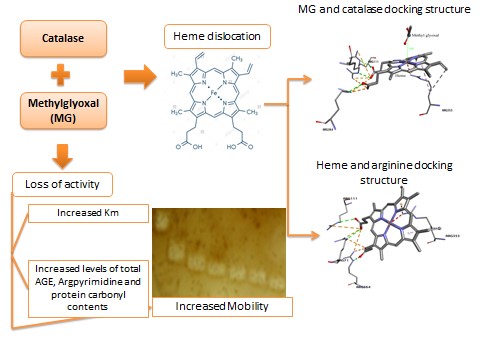
Glycation, the non-enzymatic attachment of glucose to protein, is one of the important events in the pathophysiology of diabetes mellitus, Alzheimer’s, Parkinson’s and other diseases. Methylglyoxal (MG), a dicarbonyl compound formed during glycation, monosaccharide autoxidation, and metabolism is elevated during diabetes mellitus. Among other antioxidant enzymes, catalase is important for the defense against oxidative damage. However, antioxidant enzymes including catalase can themselves become targets of non-enzymatic modification by methylglyoxal. In this study, catalase was incubated with increasing concentrations of MG for different time intervals. Structural and functional alterations to catalase were monitored by a variety of approaches, namely, assay of enzyme activity, staining of gels for activity as well as heme, measurement of protein carbonyls and Arg pyrimidine, which is a specific MG modification product. A progressive increase in electrophoretic mobility and detachment of heme from the monomer were observed with increasing concentrations of methylglyoxal. The MG-modified enzyme showed reduced affinity towards the substrate hydrogen peroxide. Molecular modeling studies revealed that MG can access the heme and arginine residues close to it. Thus, the decrease in activity of methylglyoxal-modified catalase may be important in aggravating the severity of secondary complications seen in diabetes mellitus.
HIGHLIGHTS
•Increase in concentration of methylglyoxal caused a progressive increase in electrophoretic mobility and detachment of heme from the monomer.
•MG-modified enzyme showed reduced affinity towards the substrate hydrogen peroxide.
•Molecular modeling studies showed that MG can access the heme and arginine residues close to it.
- Catalase
- Diabetes mellitus
- Glycation
- Heme
- Methylglyoxal
How to Cite
References
Aebi. H.E. (1983). ″Catalase″ In: H. U. Bergmeyer (Ed.), Methods in Enzymatic Analysis. Ed. 3rd, Werlag Chemi GmbH, Weinheim, pp. 278-282.
Ahmad, M. U., Frye, E. B., Degenhardt, T. P., Thorpe, S. R. and J. W. Baynes, (1997). ″N-(Carboxymethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. ″ Biochemical Journal. 324: 565-570.
Bakala, H., Hamelin, M., Mary, J., Borot-Laloi, C. and B. Friguet, (2012). ″Catalase, a target of glycation damage in rat liver mitochondria with aging. ″ Biochimica et Biophysica Acta. 1822:1527–1534.
Chaplen, F.W.R., Fahl, W.E., and C.C. Douglas, (1998). ″Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells.″ Proceedings of the National Academy of Sciences of the United States of America. 95: 5533-5538.
Faure, P., Troncy, L., Lecomte, M., Wiernsperger, N., Lagarde, M., Ruggiero, D. and S. Halimi, (2005). ″Albumin antioxidant capacity is modified by methylglyoxal.″ Diabetes & Metabolism. 31:169–177.
Gregory, E. M. and I. Fridovich, (1974). ″Visualization of catalase on acrylamide gels.″ Analytical Biochemistry. 58: 57-62.
Honke, K. and N. Taniguchi, (2003). ″Identification of the binding site of methylglyoxal on glutathione peroxidase: methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites Arg 184 and 185. ″ Free Radical Research. 37: 205–211.
Hoque, M. A., Uraji, M., Torii, A., Banu, M. N., Mori, I. C., Nakamura, Y. and Y. Murata, (2012). ″Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum.″ Journal of Biochemical and Molecular Toxicology. 26: 315–321.
Kasai, H., Kumeno, K., Yamaizumi, Z., Nishimura, S., Nagao, M., Fujita, Y., Nukaya, H. and T. Kosuge, (1982). ″Mutagenecity of methylglyoxal in coffee.″ GANN. 73: 681-683.
Kakkar, R., Kalra, J., Mantha, S. V. and K. Prasad, (1995). ″Lipid peroxidation and activity of antioxidant enzymes in diabetic rats.″ Molecular and Cellular Biochemistry. 151: 113-119.
Kalapos, M. (1999). ″Methylglyoxal in living organisms-Chemistry, biochemistry, toxicology and biological implications.″ Toxicology Letters. 110: 145-175.
Laemmli, U. K. (1970). ″Cleavage of structural proteins during the assembly of the head of bacteriophage T4.″ Nature. 227: 680-685.
Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A. G., Ahn, B. W., Shaltiel, S. and E.R. Stadtman, (1990). ″Determination of carbonyl content in oxidatively modified proteins.″ In: Packer L and Glazer AN (Eds). Methods in Enzymology, Academic press, London. pp. 464-478.
McLellan, A. C., Thornalley, P. J., Benn, J. and P. H. Sonksen, (1993). ″Modification of the glyoxalase system in clinical diabetes mellitus.″ Biochemical Society Transactions. 21: 158S.
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S. and A. J. Olson, (2009). ″AutoDock4 and AutodockTools4: automated docking with selective receptor flexibility.″ Journal of Computational Chemistry. 16: 2785–2791.
Oya, T., Hattory, N., Mizuno, Y., Miyata, T., Maedo, S., Osawa, T. and K. Uchida, (1999). ″Methylglyoxal modification of protein: chemical and immunochemical characterization of methylglyoxal-arginine adducts.″ Journal of Biological Chemistry. 274: 18492-18502.
Philips, S. A., Mirrlees, D. and P. J. Thornalley, (1993). ″Modification of the glyoxalase system in streptozotocin-induced diabetic rats and the effect of the aldose reductase inhibitor statil.″ Biochemical Pharmacology. 46: 805-811.
Park, Y. S., Koh, Y. H., Takahashi, M., Miyamoto, Y., Suzuki, K., Dohmae, N., Takio, K., Toleikis, P. M. and D. V. Godin, (1995). ″Alteration of antioxidant status in diabetic rats by chronic exposure to psychological stressors.″ Pharmacology Biochemistry and Behavior. 52: 355-366.
Putnam, C. D., Arvai, A. S., Bourne, Y. and J. A. Tainer, (2000). ″Active and Inhibited Human Catalase Structures: Ligand and NADPH Binding and Catalytic Mechanism.″ Journal of Molecular Biology. 296: 295-309.
Prakash, K., Prajapati, S., Ahmad, A., Jain, S. and V. Bhakuni, (2002). ″Unique oligomeric intermediates of bovine liver catalase.″ Protein Science. 11: 46-57.
Samejima, T., Kamata, M. and K. Shibata, (1962). ″Dissociation of beef liver catalase.″ Biochemical Journal. 51: 181-187.
Suji, G. and S. Sivakami, (2004). ″Glucose, glycation and aging.″ Biogerontology. 5: 365-373.
Suji, G. and S. Sivakami, (2007). ″DNA damage during glycation of lysine by methylglyoxal: assessment of vitamins in preventing damage.″ Amino Acids. 33: 615-621.
Scheckhuber, C. Q. (2015). ″Arg354 in the catalytic centre of bovine liver catalase is protected from methylglyoxal‑mediated glycation.″ BMC Research Notes. 8: Article No. 830.
Toleikis, P. M. and D. V. Godin, (1995). ″Alteration of antioxidant status in diabetic rats by chronic exposure to restraint stressors.″ Pharmacology Biochemistry Behavior. 52: 355–366.
Trott, O. and A. J. Olson, (2010). ″AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading.″ Journal of Computational Chemistry. 31: 455–446.
Uchida, K., Khor, O. T., Oya, T., Osawa, T., Yasuda, Y. and T. Miyata, (1997). ″Protein modification by a Maillard reaction intermediate methylglyoxal: Immunochemical detection of fluorescent 5-methylimidazolone derivatives in vivo.″ FEBS Letters. 410: 313-318.
Yadav, P., Sarkar, S. and D. Bhatnagar, (1997). ″Lipid peroxidation and antioxidant enzymes in erythrocytes and tissues in aged diabetic rats.″ Indian Journal of Experimental Biology. 35: 389-392.
Yan, H. and J. J. Harding, (1997). ″Glycation induced inactivation and loss of antigenicity of catalase and superoxide dismutase.″ Biochemical Journal. 328: 599-605.
- Abstract Viewed: 790 times
- PDF Downloaded: 150 times