Mutagenesis and Protoplast Fusion for Enhanced Bacteriocins Production Bacteriocins enhanced production
Applied Food Biotechnology,
Vol. 8 No. 2 (2021),
16 March 2021
,
Page 133-142
https://doi.org/10.22037/afb.v8i2.32505
Abstract
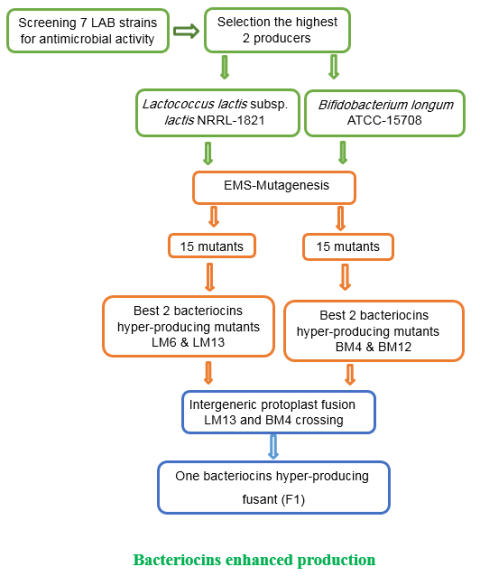
Background and Objective: Induced mutagenesis is widely used to produce novel mutants with improved productivities. Ethyl methane-sulfonate-induced mutagenesis followed by intergeneric protoplast fusion were used to develop lactic acid bacterial strains with high antimicrobial activities.
Materials and Methods:
The antagonistic activities of seven LAB strains were assessed against seven indicator microorganisms using well diffusion assay. The highest two producers were subjected to ethyl methane-sulfonate mutagenesis followed by intergeneric protoplast fusion. Selection of the mutants and the fusants from the suggested fusion cross was based on the responses to different antibiotics.
Results and Conclusion:
Lactococcus lactis subsp. lactis and Bifidobacterium longum showed the highest antimicrobial activities against most of the indicator microorganisms. Such activities were achieved at pH 2.0 and dramatically decreased by increasing the pH level. Ethyl methane-sulfonate-induced mutagenesis resulted in thirty mutants, four of which exhibited higher activities than their wild type parental strains (two for each parent). In an attempt to increase such activity, intergeneric protoplast fusions between LM 13 (resulting from Lactococcus lactis subsp. lactis) and BM 4 (resulting from Bifidobacterium longum) mutants were carried out. Twelve fusants were obtained. Interestingly, one fusant (F1) showed an increase in antimicrobial activity, compared to its parental strains. An increased range of 58.1 to 345.7% compared to the parental strain Lactococcus lactis subsp. lactis and a range of 51.5 to 168.5% for the second parental strain were noticed. The LM 13, LM 6, BM 4 and BM 12 mutants and the F1 fusant can be used in the preservation of food products.
- ▪ Bacteriocins
- ▪ Bifidobacterium longum
- ▪ Ethyl methane sulfonate
- ▪ Intergeneric protoplast fusion
- ▪ Lactococcus lactis subsp. lactis
How to Cite
References
References
Liao SF, Nyachoti CM. Using probiotics to improve swine gut health and nutrientutilization. Anim Nutr. 2017; 3:331-343.
doi: 10.1016/j.aninu.2017.06.007.
Abbasiliasi S, Tan JS, Ibrahim TAT, Bashokouh F, Rama-krishnan NR, Mustafa S, Ariff AB. Fermentation factors influencing the production of bacteriocins by lacticacid bacteria. A review. RSC Adv. 2017; 7: 29395-29420.
doi: 10.1039/c6ra24579j.
Zarrabi A, Alipoor MA, Khorasani S, Mohammadabadi M, Jamshidi A, Torkaman S, Taghavi E, Mozafari MR, Rasti B. Nanoliposomes and tocosomes as multifunctional nanoca-rriers for the encapsulation of nutraceutical and dietary molecules. Molecules 2020; 25 (3): 638-660
doi: 10.3390/molecules25030638.
Cotter PD, Ross RP, Hill C. Bacteriocins a viable alternative to antibiotics. Nat Rev Microbiol. 2012; 11:95-105.
doi:10.1038/nrmicro2937.
- de Vos WM, Kuipers OP, Van der Meer JR, Siezen RJ. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by Gram-positive bacteria. Mol. Microbiol. 1995; 17: 427-437.
doi: 10.1111/j.1365-2958.1995.mmi_17030427.x.
Ramu R, Shirahatti PS, Devi AT, Prasad A. Bacteriocins and their applications in food preservation. Food Sci Nutr. 2015; 20:1-42.
doi: 10.1080/10408398.2015.1020918.
Abdelrazak A, Elaasser MM, Elshal M, Haraun SA. Bacteriocin production by newly isolated Lactobacillus strain. J Pharm Biotechnol Sci. 2016; 11:39-49.
doi: 10.9790/30 -1103013949.
Sieiro PA, Lopez MM, Mu D, Kuiperes OP. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biotechnol. 2016; 100:2939-2951.
doi:10.1007/s00253-016-73 3-9.
Ni Z, Zhang X, Liu F, Wang M, Hao R., Ling P, Zhu X. Effect of Co-overexpression of nisin key genes on nisin production improvement in Lactococcus lactis LS01. Probiotics Antim-icro Prot. 2017; 9:204−212.
doi: 10.1007/s12602-017-9268-8.
Al-sheddy I, Al-Dagal M, Bazaraa WA. Microbial and sensory quality of fresh camel meat treated with organic acid salts and/or bifidobacteria. J Food Sci.1999; 64: 336-339.
doi:. 10.1111/j.1365-2621. 1999. tb15895.x
da Costa RJ, Voloski FLS, Mondadori RG, Duval EH, Fiorentini AM. Preservation of meat products with bacterio-cins produced by lactic acid bacteria isolated from meat. J Food Qual. 2019; 11:1-12.
doi:10.1155/2019/4726510.
Silva CG, Sovia PMS, Ribeiro SC. Application of bacteriocins and protective cultures in dairy food preservation. Front Microbiol. 2018; 9:1-15.
doi: 10.3389/fmicb.2018.00594
Acuna L, Carbalan N, Baluja MQ, Velazquez JB, Bellomio A. Expression of the hybrid bacteriocin Ent 35-Mccv in Lactococcus lactis and its use for controlling Listeria monocytogenes and Escherichia coli in milk. Int Dairy J. 2020; 104:125-131.
doi: 10.1016/j.idairyj.2020.104650
Al-Dagal MM, Bazaraa WA. Extension of shelf life of whole and peeled shrimp with organic acids salts and bifidobacteria. J Food Prot. 1999; 62: 51−56.
doi:10.4315/0362-028x-62.1.51.
Bazaraa WA, Abdel Aziz ME, Goda HA, Addel Kader SN. Biopreservation of the fresh Egyptian nile perch fillets using combination of bacteriocins, sodium acetate and EDTA. Biosci Res. 2019; 16:1060-1075.
Linares-Morales JR, Gutierrez-Mendez N, Rivera-Chavira BE, Perez-Vega SB, Nevarez-Moorillon GV. Biocontrol proce-sses in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front Sustain Food Syst. 2018; 50: 1-13.
doi: 10.3389/fsufs.2018.00050
Aloui H, Khwaldia K. Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Comp Rev Sci Food Safety. 2016; 15: 1080-1103.
doi:10.1111/1541-4337.12226.
Hammami R, Fliss T, Corsetti A. Application of protective cultures and bacteriocins for food biopreservation. Front Microbiol. 2019; 10:1−2.
doi: 10.3389/fmicb.2019.01561
Leja K, Myszka AK, Czaczyk K. Genome shuffling: a method to improve biotechnological processes. J Biotechnol. 2011; 92:345-351.
doi: 10.5114/bta.2011.46551
Khattab AA. Molecular and biochemical studies of genetically constructed lactic acid bacteria, Ph.D. Thesis, Tanta University, Egypt. 2002.
Khattab AA, Ibrahim MIM, EL-Kady AA. Ochratoxin a biosorption on to genetically improved of Lactobacillus delbrueckii mutants. Int Food Res J. 2018; 25(2): 515-522.
Biot-Pelletier D, Martin VJ. Evolutionary engineering by genome shuffling. Appl Microbiol Biotechnol. 2014; 98:3877-3887.
doi: 10.1007/s00253-014-5616-8.
John RP, Nampoothiri KM. Strain improvement of Lactobacillus delbrueckii using nitrous acid mutation for L-lactic acid production. World J Microbiol Biotechnol. 2008; 24: 3105−3109.
doi: 10.1007/s11274-008-9826-z
Almalk AM. Production of medically important lactic acid by Lactobacillus pentosus: A biological conversion method. Indian J Sci Technol. 2016; 9: 2-7.
doi: 10.17485/ijst/2016/v9i4/84143.
Sharma N, Prakash A. Effect of UV and microwave irradiations on Lactobacillus fermentum. IIS Univ J Sci Technol. 2015; 4: 11-18.
Hu W, Chen J, Wu Q, Li W, Liu J, Lu D, Wang S. The mutagenesis of Lactobacillus thermophilus for enhanced L- (+)-lactic acid accumulation induced by heavy ion irradiation. Braz Arch Biol Technol. 2017; 60: 1-11.
doi: 10.1590/1678-4324-2016160337.
Abddallah NA, Hussein HA, Khalil OA, Aal SA, El desokey RA. Enhancement of lactic acid bacteria by Gamma radiation to inhibit antibiotic resistance of some Salmonella spp. Arab J Nucl Sci Appl. 2018; 51: 100-107.
doi:10.21608/AJNSA.2018.12403.
Heidarpour F, Mohammadabadi MR, Zaidul ISM, Maherani B, Saari N, Hamid AA, Abas F, Manap MYA, Mozafari MR. Use of prebiotics in oral delivery of bioactive compounds: A nanotechnology perspective. Pharmazie 2011; 66 (5): 319-324.
doi: 10.1691/ph.2011.0279
Wang Y, Li Y, Pei X, Yu L, Feng Y. Genome-shuffling improved acid tolerance and L-lactic acid volumetric productivity in Lactobacillus rhamnosus. J Biotechnol. 2007; 129:510-515.
doi: 10.1016/j.jbiotec.2007.01.011.
Dahikar SB, Bhutada SA. Genome shuffling of Lactobacillus rhamnosus for improved production of lactic acid. Int Biol Res. 2017; 2:142-146.
Thomas BT, Agu GC, Makanjuola SO, Popoola OD. Genome shuffling of Lactobacillus fermentum for improved production of lactic acid. American J Res Comm. 2014; 2: 245-250.
Singhvi M, Gurjar G, Gupta V, Gokhale D. Biocatalyst development for lactic acid production at acidic pH using inter-generic protoplast fusion. RSC Adv. 2015; 5: 2024-2031.
doi: 10.1039/c4ra11104d
Wang H, Sun Y, Chen C, Sun Z, Zhou YC, Shen F, Zhang H, Dai Y. Genome shuffling of Lactobacillus plantarum for improving antifungal activity. Food Control. 2013; 32: 341-347.
doi: 10.1016/j.foodcont.2012.12.020
Kirby-Bauer A. Susceptibility test with single, high-concentration antimicrobial discs. Antimicrob Agents Chemother. 1979; 3:324-418.
Lee-Wickner LJ, Chassy B. Production and regeneration of Lactobacillus casei protoplasts. Appl Environ Microbiol. 1984; 48: 994-1000.
doi: 10.1128/AEM.48.5.994-1000.1984
Efthymiou C, Hansen PA. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962; 110: 258-267.
doi:10.1093/infdis/110.3.258.
Chassy MB. Prospects for the genetic manipulation of Lactobacilli. FEMS Microbiol Lett. 1987; 46: 297-312.
doi:10.1111/j.1574-6968. 1987.tb02467.x.
Snedecor GW, Cochran WG. Statistical Methods. 7th edition. Ames (IA): Iowa State University Press. 1980. pp: 237-252
Nomoto K. Prevention of infections by probiotics. J Biosci Bioeng. 2005; 100: 583-592.
doi: 10.1263/jbb.100.583.
Sarika AR, Lipton AP, Aishwarya MS, Dhivya RS. Efficacy of bacteriocin of Enterococcus faecalis CD1 as a biopre-servative for high value marine fish reef cod (Epinephelus diacanthus) under different storage conditions. J Microbiol Biotechnol Res. 2011; 1:18-24.
Raichurkar SJ, Athawale GH. Biopreservative: Bacteriocin its classification and applications in food. Food Sci Res J. 2015; 6: 363-374.
doi:10.15740/HAS/FSRJ/6.2/363-374
Rabie MA, Moustafa MG, Abdel-Wahed EM, Al-Harby BK, El-Zahar K. Bacteriocin-like substances produced by specific strains of lactic acid bacteria isolated from milk products. J Microbiol. 2018; 13: 70-83.
doi: 10.3923/jm.2018.70.83
Joshi VK, Sharma S, Rana NS. Production, purification, stability and efficacy of bacteriocin from isolates of natural lactic acid fermentation of vegetables. Food Technol Biotechnol. 2006; 44: 435-439.
Khan A, Vu KD, Riedl B, Lacroix M. Optimization of the antimicrobial activity of nisin, Na-EDTA and pH against Gram negative and Gram-positive bacteria. LWT Food Sci Technol. 2015; 61: 124-129.
doi: 10.1016/j.lwt.2014.11.035
Adesina IA, Enerijiofi KE. Effect of pH and heat treatment on bacteriocin activity of Pediococcus pentosaceus IO1, Tetragenococcus halophilus PO9 and Lactobacillus cellobiosus BE1. Sau Sci Tech J. 2016; 1:113-118.
Margino S, Winarti S, Indrati R, Rahayu SE. Mutation technique for increasing the production of antibacterial Lactobacillus plantarum TGR-2. Indonesian Food Nutr Progr. 1998; 5:73-78.
doi: 10.22146/jifnp.71
Hussein MA. Strain improvement of Lactobacillus plantarum for production of bacteriocin and their purification and characterization. Eur J Biotechnol Biosci. 2017; 5: 59-62.
Stein T. Oxygen-limiting growth conditions and deletion of the transition state regulator protein Abrb in Bacillus subtilis 6633 result in an increase in subtilosion production and a decrease in subtilisin production. Probiotics Antimicrob Proteins 2020; 12(2): 725-731.
doi: 10.1007/s12602-019-09547-4.
Zhang YF, Liu SY, Du YH, Feng WJ, Liu JH, Qiao JJ. Genome shuffling of Lactococcus lactis subspecies lactis YF11 for improving nisin Z production and comparative analysis. J Dairy Sci. 2014; 97: 2528-2541.
doi: 10.3168/jds.2013-7238
- Abstract Viewed: 640 times
- pdf Downloaded: 513 times
