Yeast-Lactobacillus Co-Cultures as in situ Ethanol Producers for Flavor Ester Synthesis using Lipase in Fermented Milks
Applied Food Biotechnology,
Vol. 8 No. 2 (2021),
16 March 2021
,
Page 151-160
https://doi.org/10.22037/afb.v8i2.30873
Abstract
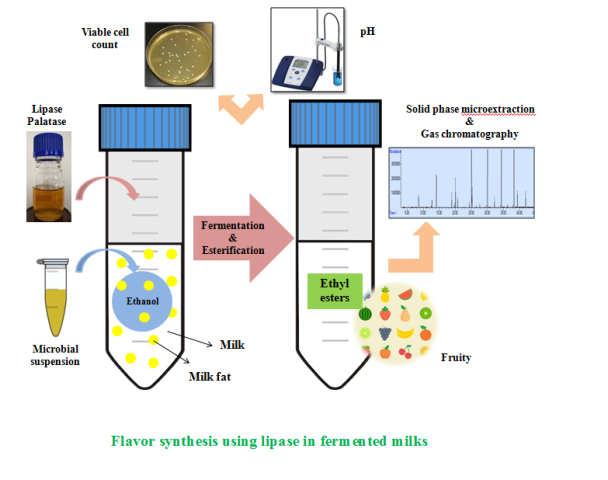
Background and objective: Nowadays, novel biotechnological methods are preferred for flavoring productions since traditional methods include disadvantages. The aim of this study was to assess in situ biosynthesis of natural fruity flavors in fermented milks using microbial co-cultures and lipase enzyme.
Materials and methods:
Trans esterification of milk fats with ethanol was carried out to develop fruity flavors in ultra-high-temperature whole milks using lipase of Palatase coupled with ethanol fermentation. Kluyveromyces marxianus NCYC 1425 was used to produce in situ ethanol in co-cultures with Lactobacillus fermentum PCC or Lactobacillus paracasei L26. Effects of co-culturing on cell viability and fermentation progress were assessed using enumeration of viable cells and measurement of pH in samples at 0, 24 (Palatase addition) and 48 h (end of fermentation). Headspace solid phase microextraction-gas chromatography (SPME)-MS/FID was used for ester, ethanol and free fatty acid analyses at the beginning and end of the fermentation. Standard curve of ethanol was used to assess the products in terms of being Halal.
Results and conclusion:
Kluyveromyces marxianus included synergistic effects on Lactobacillus paracasei growth as well as antagonistic effects on Lactobacillus fermentum growth. Antimicrobial effects were seen in Kluyveromyces marxianus-Lactobacillus paracasei co-cultures when Palatase was added. Palatase significantly increased ester levels of the fermented samples. The co-cultures did not include significant differences in shorter chain ester levels (esters of 4-7 carbon chain fatty acids); in contrast, Kluyveromyces marxianus- Lactobacillus fermentum resulted in higher levels of longer chain esters. Although the Kluyveromyces marxianus cultures resulted in higher ester levels compared to that its co-cultures did, the cultures can be used as appropriate adjunct cultures with Lactobacillus cultures to boost flavor ester synthesis. This flavor synthesis can be an appropriate alternative for artificial flavoring agents.
- Fermented milk
- Flavor ester
- Kluyveromyces marxianus
- Lactobacillus
- Lipase
- Solid phase microextraction (SPME)
How to Cite
References
Carocho M, Morales P, Ferreira IC. Natural food additives: Quo vadis?. Trends Food Sci Tech. 2015;45(2):284-295.
https://doi.org/10.1016/j.tifs.2015.06.007
Chaudhary NK. Food additives. Bibechana. 2010;6:22-26.
https://doi.org/10.3126/bibechana.v6i0.3935
Bader J, Mast‐Gerlach E, Popović MK, Bajpai R, Stahl U. Relevance of microbial coculture fermentations in biotechnology. J Appl Microbiol. 2010;109:371-387.
https://doi.org/10.1111/j.1365-2672.2009.04659.x
Vandamme EJ, Soetaert W. Bioflavours and fragrances via fermentation and biocatalysis. J Chem Technol Biot. 2002;77(12):1323-1332.
https://doi.org/10.1002/jctb.722
Longo MA, Sanromán MA. Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotech. 2006;44(3):335-353.
Ferreira-Dias S, Sandoval G, Plou F, Valero F. The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron J Biotechno. 2013;16(3):1-24.
http://doi.org/10.2225/vol16-issue3-fulltext-5
Liu SQ, Lee HY, Yu B, Curran P, Sun J. Bioproduction of natural isoamyl esters from coconut cream as catalysed by lipases. J Food Res. 2013;2(2):157-166.
http:// doi.org/10.5539/jfr.v2n2p157
Liu SQ, Holland R, Crow V. Synthesis of ethyl butanoate by a commercial lipase in aqueous media under conditions relevant to cheese ripening. J Dairy Res. 2003;70:359-363.
https://doi.org/10.1017/S0022029903006290
Sun J, Yu B, Curran P, Liu SQ. Optimisation of flavour ester biosynthesis in an aqueous system of coconut cream and fusel oil catalysed by lipase. Food Chem. 2012;135(4):2714-2720.
https://doi.org/10.1016/j.foodchem.2012.06.119
Liu SQ, Holland R, Crow VL. Esters and their biosynthesis in fermented dairy products: a review. Int Dairy J. 2004;14(11):923-945.
https://doi.org/10.1016/j.idairyj.2004.02.010
Lindmark Månsson H. Fatty acids in bovine milk fat. Food Nutr Res. 2008;52(1):1821-1823
https://doi.org/10.3402/fnr.v52i0.1821
Liu SQ, Crow VL, Holland R. Production of natural fruity flavour in dairy foods. Nutr Food Sci. 2009;39(5):483-489.
http://doi.org/10.1108/00346650910992132
Zhang XM, Ai NS, Wang J, Tong LJ, Zheng FP, Sun BG. Lipase-catalyzed modification of the flavor profiles in recombined skim milk products by enriching the volatile components. J Dairy Sci. 2016;99(11):8665-8679.
http://doi.org/10.3168/jds.2015-10773
Sun J, Lim Y, Liu SQ. Biosynthesis of flavor esters in coconut cream through coupling fermentation and lipase-catalyzed biocatalysis. Eur J Lipid Sci Tech. 2013;115(10):1107-1114.
https://doi.org/10.1002/ejlt.201300144
Silveira WB, Passos FJ, Mantovani HC, Passos FM. Ethanol production from cheese whey permeate by Kluyveromyces marxianus UFV-3: a flux analysis of oxido-reductive metabolism as a function of lactose concentration and oxygen levels. Enzyme MicrobTech. 2005;36(7):930-936.
https://doi.org/10.1016/j.enzmictec.2005.01.018
Medeiros AB, Pandey A, Freitas RJ, Christen P, Soccol CR. Optimization of the production of aroma compounds by Kluyveromyces marxianus in solid-state fermentation using factorial design and response surface methodology. Biochem Eng J. 2000;6(1):33-39.
https://doi.org/10.1016/S1369-703X(00)00065-6
Bernardeau M, Vernoux JP, Henri-Dubernet S, Gueguen M. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol. 2008;126(3):278-285.
https://doi.org/10.1016/j.ijfoodmicro.2007.08.015
Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. App Microbiol Biot. 2010;85(6):1629-1642.
https://doi.org/10.1007/s00253-009-2355-3
Pohl CH, Kock JL, Thibane VS. Antifungal free fatty acids: a review. Science against microbial pathogens: communicating current research and technological advances. 2011;1:61-71.
Basso TO, Gomes FS, Lopes ML, de Amorim HV, Eggleston G, Basso LC. Homo-and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. A Van Leeuw J Microb. 2014;105(1):169-177.
https://doi.org/10.1007/s10482-013-0063-6
Aksu Z, Dönmez G. The use of molasses in copper (II) containing wastewaters: effects on growth and copper (II) bioaccumulation properties of Kluyveromyces marxianus. Process Biochem. 2000;36(5):451-458.
https://doi.org/10.1016/S0032-9592(00)00234-X
Stadie J, Gulitz A, Ehrmann MA, Vogel RF. Metabolic activity and symbiotic interactions of lactic acid bacteria and yeasts isolated from water kefir. Food Microbiol. 2013;35:92-98.
https://doi.org/10.1016/j.fm.2013.03.009
Narendranath NV, Thomas KC, Ingledew WM. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biot. 2001,26:171-177.
https://doi.org/10.1038/sj.jim.7000090
Bayrock DP, Ingledew WM. Inhibition of yeast by lactic acid bacteria in continuous culture: nutrient depletion and/or acid toxicity?. J Ind Microbiol Biot. 2004;31(8):362-368.
https://doi.org/10.1007/s10295-004-0156-3
Toh M, Liu SQ. Impact of co-culturing Bifidobacterium animalis subsp. lactis HN019 with yeasts on microbial viability and metabolite formation. J Appl Microbiol. 2017;123(4):956-968.
https://doi.org/10.1111/jam.13571
Thomas KC, Hynes SH, Ingledew WM. Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J Appl Microbiol. 2001;90:819-828.
https://doi.org/10.1046/j.1365-2672.2001.01311.x
Annan NT, Poll L, Sefa‐Dedeh S, Plahar WA, Jakobsen M. Volatile compounds produced by Lactobacillus fermentum, Saccharomyces cerevisiae and Candida krusei in single starter culture fermentations of Ghanaian maize dough. J Appl Microbiol. 2003;94(3):462-474.
https://doi.org/10.1046/j.1365-2672.2003.01852.x
Quigley L, O'Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. The complex microbiota of raw milk. FEMS microbiology reviews. 2013;37(5):664-698.
https://doi.org/10.1111/1574-6976.12030
Jensen RG, Galluzzo DR, Bush VJ. Selectivity is an important characteristic of lipases (acylglycerol hydrolases). Biocatalysis. 1990;3(4):307-316.
https://doi.org/10.3109/10242429008992074
Kurtovic I, Marshall SN, Miller MR, Zhao X. Flavour development in dairy cream using fish digestive lipases from Chinook salmon (Oncorhynchustshawytscha) and New Zealand hoki (Macruronusnovaezealandiae). Food Chem. 2011;127(4):1562-1568.
https://doi.org/10.1016/j.foodchem.2011.02.018
Tan HS, Yu B, Curran P, Liu SQ. Lipase-catalysed synthesis of natural aroma-active 2-phenylethyl esters in coconut cream. Food Chem. 2011;124(1):80-84.
https://doi.org/10.1016/j.foodchem.2010.05.108
Mason AB, Dufour JP. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast. 2000;16(14):1287-1298.
https://doi.org/10.1002/1097-0061(200010)16:14<1287::AID-YEA613>3.0.CO;2-I
Martinez FA, Balciunas EM, Salgado JM, González JM, Converti A, de Souza Oliveira RP. Lactic acid properties, applications and production: a review. Trends Food Sci Tech. 2013;30(1):70-83.
https://doi.org/10.1016/j.tifs.2012.11.007
Alzeer J, Hadeed KA. Ethanol and its Halal status in food industries. Trends Food Sci Tech. 2016;58:14-20.
https://doi.org/10.1016/j.tifs.2016.10.018
Chen LD, Daniel RM, Coolbear T. Detection and impact of protease and lipase activities in milk and milk powders. Int Dairy J. 2003;13(4):255-275.
https://doi.org/10.1016/S0958-6946(02)00171-1
Brindisi JA, Parker JD, Turner LG, Larick DK. Chemical profiles of hydrolyzed milk samples after treatment with commercial enzymes. J Food Sci. 2001;66(8):1100-1107.
- Abstract Viewed: 567 times
- pdf Downloaded: 573 times
