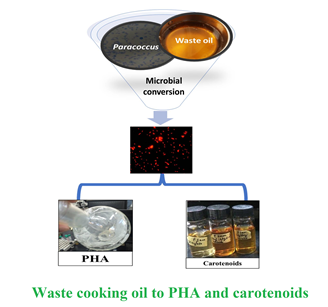
Paracoccus sp. Strain LL1 as a Single Cell Factory for the Conversion of Waste Cooking Oil to Polyhydroxyalkanoates and Carotenoids
Applied Food Biotechnology,
Vol. 6 No. 1 (2019),
2 January 2019
,
Page 53-60
https://doi.org/10.22037/afb.v6i1.21628
Abstract
Background and objective: Polyhydroxyalkanoates have drawn significant attention as alternative to petroleum-based plastics; however, their industrial production is still hindered by the costly feed materials. Co-generation of other high-value products in addition to polyhydroxyalkanoate by the same microbial strains can be helpful in alleviating overall production cost up to 50%. This study for the first time demonstrates that polyhydroxyalkanoate and astaxanthin-rich carotenoids can be co-produced by Paracoccus sp. LL1 using waste cooking oil as substrate.
Material and methods: The halophilic strain of Paracoccus sp. LL1 was grown under batch fermentation using mineral media supplemented with 1% (v v-1) waste cooking oil. Different surfactants were used to improve substrate utilization. Polyhydroxyalkanoate obtained after the fermentation was characterized by fluorescent microscopy, gas chromatography, and Fourier Transform Infra-Red spectroscopy.
Results and conclusion: Oil as a substrate, led to 1.0 g l-1 poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with concomitant production of 0.89 mg l-1 of carotenoids after 96 h. An enhancement of 2.7-folds in total cell dry mass was achieved when 0.1% (v v-1) Tween-80 was used as surfactant for ease in oil metabolism. Paracoccus sp. LL1 has the potential to serve as a single cell factory for bioconversion of cheap substrates into high value products.
Conflict of interest: The authors declare no conflict of interest.
- ▪ Astaxanthin ▪ Co-production ▪ Polyhydroxyalkanoates ▪ Vegetable oil ▪ Waste cooking
How to Cite
References
Kumar P, Patel SKS, Lee J-K, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013; 31(8): 1543-1561. doi: 10.1016/j.biotechadv.2013.08.007
Poltronieri P, Kumar P. Polyhydroxyalkanoates (PHAs) in Industrial Applications. In: Martinez LMT, Kharissova OV, Kharisov BI, editors. Handbook of Ecomaterials. Cham: Springer International Publishing; 2017: pp. 1-30. doi: 10.1007/978-3-319-48281-1_70-1
Kumar P, Singh M, Mehariya S, Patel SKS, Lee J-K, Kalia VC. Ecobiotechnological approach for exploiting the abilities of Bacillus to produce co-polymer of polyhydroxyalkanoate. Indian J Microbiol. 2014; 54(2): 151-157. doi: 10.1007/s12088-014-0457-9
Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC. Biotechnology in Aid of Biodiesel Industry Effluent (glycerol): Biofuels and Bioplastics. In: Kalia VC, editor. Microbial Factories: Biofuels, Waste Treatment: Volume 1. New Delhi: Springer India; 2015: pp. 105-119. doi: 10.1007/978-81-322-2598-0_7
Ray S, Kalia VC. Co-metabolism of substrates by Bacillus thuringiensis regulates polyhydroxyalkanoate co-polymer composition. Bioresour Technol. 2017; 224: 743-747. doi: 10.1016/j.biortech.2016.11.089
Kaur G, Roy I. Strategies for large-scale production of polyhydroxyalkanoates. Chem Biochem Eng Q. 2015; 29(2): 157-272. doi: 10.15255/CABEQ.2014.2255
Kourmentza C, Placido J, Venetsaneas N, Burniol-Figols A, Varrone C, Gavala HN, Reis MA. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering. 2017; 4(2): 55. doi: 10.3390/bioengineering4020055
Koller M, Marsalek L, de Sousa Dias MM, Braunegg G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017; 37: 24-38. doi: 10.1016/j.nbt.2016.05.001
Bhatia SK, Kim J-H, Kim M-S, Kim J, Hong JW, Hong YG, Kim H-J, Jeon J-M, Kim S-H, Ahn J, Lee H, Yang Y-H, Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst Eng. 2018; 41(2): 229-235. doi: 10.1007/s00449-017-1861-4
Kumar P, Jun H-B, Kim BS. Co-production of polyhydroxyalkanoates and carotenoids through bioconversion of glycerol by Paracoccus sp. strain LL1. Int J Biol Macromol. 2018; 107: 2552-2558. doi: 10.1016/j.ijbiomac.2017.10.147
Koller M, Muhr A, Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014; 6: 52-63. doi: 10.1016/j.algal.2014.09.002
Kumar P, Chandrasekhar K, Kumari A, Sathiyamoorthi E, Kim BS. Electro-fermentation in aid of bioenergy and biopolymers. Energies 2018; 11(2): 343. doi: 10.3390/en11020343
Singh LK, Dhasmana N, Sajid A, Kumar P, Bhaduri A, Bharadwaj M, et al. clpC operon regulates cell architecture and sporulation in Bacillus anthracis. Environ Microbiol. 2015;17 (3):855-865. doi: 10.1111/1462-2920.12548
Costa SGVAO, Lepine F, Milot S, Deziel E, Nitschke M, Contiero J. Cassava wastewater as a substrate for the simultaneous production of rhamnolipids and polyhydroxyalkanoates by Pseudomonas aeruginosa. J Ind Microbiol Biotechnol. 2009; 36(8): 1063-1072. doi: 10.1007/s10295-009-0590-3
Kumar P, Mehariya S, Ray S, Mishra A, Kalia VC. Biodiesel industry waste: A potential source of bioenergy and biopolymers. Indian J Microbiol. 2015; 55(1): 1-7. doi: 10.1007/s12088-014-0509-1
Yousuf RG, Winterburn JB. Waste date seed oil extract as an alternative feedstock for Poly (3-hydroxybutyrate) synthesis. Biochem Eng J. 2017; 127: 68-76. doi: 10.1016/j.bej.2017.08.007
Kourmentza C, Costa J, Azevedo Z, Servin C, Grandfils C, De Freitas V, Ries MAM. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour Technol. 2018; 247: 829-837. doi: 10.1016/j.biortech.2017.09.138
Marsudi S, Unno H, Hori K. Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 2008; 78(6): 955-961. doi: 10.1007/s00253-008-1388-3
Cui Y-W, Gong X-Y, Shi Y-P, Wang Z. Salinity effect on production of PHA and EPS by Haloferax mediterranei. RSC
Adv. 2017; 7(84): 53587-53595. doi: 10.1039/C7RA09652F 20. Obruca S, Petrik S, Benesova P, Svoboda Z, Eremka L, Marova I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl Microbiol Biotechnol. 2014; 98(13): 5883-5890. doi: 10.1007/s00253-014-5653-3
Sawant SS, Salunke BK, Kim BS. Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp. LL1. Bioresour Technol. 2015; 194: 247-255. doi: 10.1016/j.biortech.2015.07.019
Kumar P, Ray S, Patel SKS, Lee J-K, Kalia VC. Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol. 2015; 78: 9-16. doi: 10.1016/j.ijbiomac.2015.03.046
Kumar P, Ray S, Kalia VC. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour Technol. 2016; 200: 413-419. doi: 10.1016/j.biortech.2015.10.045
Song JH, Jeon CO, Choi MH, Yoon SC, Park W. Polyhydroxyalkanoate (PHA) production using waste vegetable oil by Pseudomonas sp. strain DR2. J Microbiol Biotechnol. 2008; 18(18): 1408-1415
Vastano M, Casillo A, Corsaro MM, Sannia G, Pezzella C. Production of medium chain length polyhydroxyalkanoates from waste oils by recombinant Escherichia coli. Eng Life Sci. 2015; 15(7): 700-709. doi: 10.1002/elsc.201500022
Verlinden RA, Hill DJ, Kenward MA, Williams CD, Piotrowska-Seget Z, Radecka IK. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express. 2011; 1(1): 11. doi: 10.1186/2191-0855-1-11
Ciesielski S, Możejko J, Pisutpaisal N. Plant oils as promising substrates for polyhydroxyalkanoates production. J Clean Prod. 2015; 106: 408-421. doi: 10.1016/j.jclepro.2014.09.040
Mozejko J, Przybylek G, Ciesielski S. Waste rapeseed oil as a substrate for medium chain length polyhydroxyalkanoates
production. Eur J Lipid Sci Technol. 2011; 113(12): 1550-1557. doi: 10.1002/ejlt.201100148
Benesova P, Kucera D, Marova I, Obruca S. Chicken feather hydrolysate as an inexpensive complex nitrogen source for PHA production by Cupriavidus necator on waste frying oils. Lett Appl Microbiol. 2017; 65(2): 182-188. doi: 10.1111/lam.12762
Campanari S, Augelletti F, Rossetti S, Sciubba F, Villano M, Majone M. Enhancing a multi-stage process for olive oil mill wastewater valorization towards polyhydroxyalkanoates and biogas production. Chem Eng J. 2017; 317: 280-289. doi: 10.1016/j.cej.2017.02.094
Cruz MV, Sarraguca MC, Freitas F, Lopes JA, Reis MAM. Online monitoring of P(3HB) produced from used cooking oil with near-infrared spectroscopy. J Biotechnol. 2015; 194:1-9. doi: 10.1016/j.jbiotec.2014.11.022
Mozejko J, Ciesielski S. Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J Biosci Bioeng. 2013; 116(4): 485-492. doi: 10.1016/j.jbiosc.2013.04.014
Obruca S, Benesova P, Oborna J, Marova I. Application of protease-hydrolyzed whey as a complex nitrogen source to increase poly (3-hydroxybutyrate) production from oils by Cupriavidus necator. Biotechnol Lett. 2014; 36(4): 775-781. doi: 10.1007/s10529-013-1407-z
Follonier S, Goyder MS, Silvestri A-C, Crelier S, Kalman F, Riesen R, Zinn M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain length polyhydroxyalkanoates. Int J Biol Macromol. 2014; 71: 42-52. doi: 10.1016/j.ijbiomac.2014.05.061
Nielsen C, Rahman A, Rehman AU, Walsh MK, Miller CD. Food waste conversion to microbial polyhydroxyalkanoates. Microb Biotechnol. 2017; 10(6): 1338-1352. doi: 10.1111/1751-7915.12776
- Abstract Viewed: 1078 times
- PDF Downloaded: 954 times